How do you manage verification and validation in the development process? Does the design of your medical device or system meet the defined specifications and requirements? Can it move on to the next phase of the development process or market launch? As a medical device manufacturer, your goal is a fast and efficient development process, leading to a shorter time-to-market. A well-planned design verification strategy is essential for a smooth and successful compliance process.
Let us work together to ensure that your verification and validation process is efficient and complies with regulatory requirements - we look forward to answering your questions in an initial, non-binding conversation!
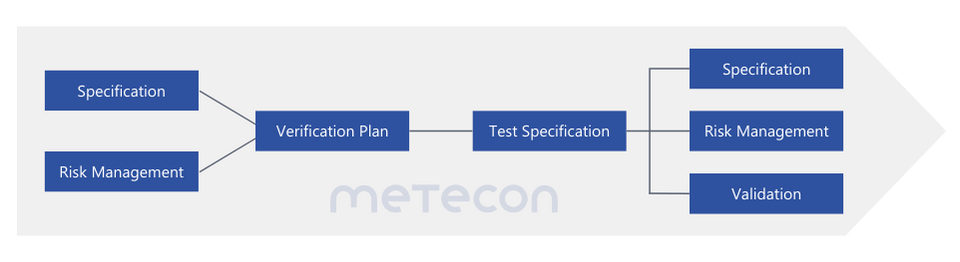
The strategic planning of your design verification
- ensures the availability of suitable test samples to fulfill regulatory requirements efficiently.
- ensures that your test samples are valid and compliant so that all results meet the applicable regulations.
- minimizes delays and saves valuable time in the development process.
- optimizes your development costs by avoiding unnecessary repetition and additional testing.
- establishes a solid foundation for seamless validation, supporting the regulatory approval process.
Let us work together to ensure that your verification and validation process is efficient and complies with regulatory requirements - we look forward to answering your questions in an initial, non-binding conversation!
Our services for the verification and validation of medical devices
A thorough verification and validation (V&V) is essential to demonstrate the safety and performance of a medical device and to meet regulatory requirements. Whether it’s development, documentation updates, or preclinical testing, we support you with the right testing strategy, precise documentation and efficient processes to optimize your time-to-market.
With our expertise, we ensure seamless traceability and an organized Design History File (DHF).Requirement gathering
We assist you in identifying and documenting (regulatory) requirements and provide guidance on formulating these requirements with regard to the demonstration of evidence.Documentation updates
Your products have been on the market for a long time and have been successful, but the documentation is not up to date?
Regulatory requirements are constantly changing and expanding, and employees come and go, taking their expertise with them. As a result, it can become necessary to update the documentation for the specification (requirements and functional specification), V&V plans and reports, risk management, or even validations. With your product expertise and our documentation experience, we will jointly create a structured Design History File (DHF).Traceability
Clear traceability - from the justification of the rationale behind a specification to the test protocols for development results and back to the validation report —offers significant advantages and is a formal requirement in certain markets. We organize requirements, verifications, validations, risks, and related evidence, linking them in an overview so that you always maintain control and ensure all elements are traceable.Preclinical Testing
Before you can bring your product to market or begin clinical trials, various tests based on the state of the art (SOTA) are required for the products. We are happy to guide and support you through this process by assisting with:
Verification of evidence
As part of a comprehensive document review, we can assess the following documents, check for plausibility and completeness, and recommend adjustments:
Design History File (DHF) and more
Let’s ensure your medical devices meet regulatory standards and are market-ready. Contact us for a free, initial consultation!
With our expertise, we ensure seamless traceability and an organized Design History File (DHF).Requirement gathering
We assist you in identifying and documenting (regulatory) requirements and provide guidance on formulating these requirements with regard to the demonstration of evidence.Documentation updates
Your products have been on the market for a long time and have been successful, but the documentation is not up to date?
Regulatory requirements are constantly changing and expanding, and employees come and go, taking their expertise with them. As a result, it can become necessary to update the documentation for the specification (requirements and functional specification), V&V plans and reports, risk management, or even validations. With your product expertise and our documentation experience, we will jointly create a structured Design History File (DHF).Traceability
Clear traceability - from the justification of the rationale behind a specification to the test protocols for development results and back to the validation report —offers significant advantages and is a formal requirement in certain markets. We organize requirements, verifications, validations, risks, and related evidence, linking them in an overview so that you always maintain control and ensure all elements are traceable.Preclinical Testing
Before you can bring your product to market or begin clinical trials, various tests based on the state of the art (SOTA) are required for the products. We are happy to guide and support you through this process by assisting with:
- comparing and selecting accredited laboratories (e. g., for EMC, electrical, or biological safety testing),
- preparing for test readiness,
- adressing corrective actions,
- supporting testing during development.
Verification of evidence
As part of a comprehensive document review, we can assess the following documents, check for plausibility and completeness, and recommend adjustments:
- specification validation,
- transport validation,
- packaging validation,
- usability validation,
- software validation,
- process validation (e.g., sterilization validation).
Design History File (DHF) and more
- Are you in conflict with your notified body / testing laboratory over "allegedly" missing documents or do you need support with your argumention?
- Have you had significant staff turnover and need to quickly train new staff as quickly as possible?
- Do your development process and records not quite align?
Let’s ensure your medical devices meet regulatory standards and are market-ready. Contact us for a free, initial consultation!
Verification and validation with Metecon: faster, safer, more flexible!
- You have your testing equipment and test specifications available on time.
- The necessary resources for development-related testing are known.
Verification can quickly become time-consuming and costly. Through strategic coordination between development and regulatory affairs, we achieve the optimal solution.
The verification process at a glance