In vitro diagnostics (IVD) are medical devices that are regulated in their own regulation (Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR)) due to their special nature:The definition of in vitro diagnostics is laid down precisely in the IVDR, Art. 2. (2):
"In vitro diagnostic medical device means a medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, device, software, or system, individually or in combination, intended by the manufacturer to be used for the in vitro examination of specimens, including blood and tissue donations, derived from the human body, exclusively or principally to provide information on one or more of the following..."Safe and reliable products should result from the efficient interaction of the various IVD quality management processes. Safety and performance of the products must be monitored and confirmed throughout the entire product life cycle. In the technical documentation, manufacturers provide proof to third parties that these processes are carried out correctly and in compliance with the respective target country.We help you meet your regulatory requirements at all levels and at any point in the product lifecycle.
Learn more and book a free appointment with us.
"In vitro diagnostic medical device means a medical device that is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, device, software, or system, individually or in combination, intended by the manufacturer to be used for the in vitro examination of specimens, including blood and tissue donations, derived from the human body, exclusively or principally to provide information on one or more of the following..."Safe and reliable products should result from the efficient interaction of the various IVD quality management processes. Safety and performance of the products must be monitored and confirmed throughout the entire product life cycle. In the technical documentation, manufacturers provide proof to third parties that these processes are carried out correctly and in compliance with the respective target country.We help you meet your regulatory requirements at all levels and at any point in the product lifecycle.
Learn more and book a free appointment with us.
New transition periods
With Regulation (EU) 2024/1860 dated June 13, 2024, the EU Commission gives manufacturers more time to transition to Regulation (EU) 2017/746 (IVDR) under certain conditions. Stay on top of all the changes.Do you have any questions? We look forward to contributing to your team's success.
Our services for your IVD
Creation and maintenance of the technical documentation:
Revision and adjustment of the quality management system:
Other services:
Please contact us to learn more about how we can help you with your IVD.
- checking the technical documentation of in-vitro diagnostics already on the market, i.e. "legacy devices" and "old devices" against the regulatory requirements,
- creation of the content of the technical documentation (EU and international) - in compliance with standards, laws, and regulations for existing products and also in the context of new developments,
- communication with Notified Bodies, authorities, and test laboratories for analytical and/or clinical studies,
- review/adjustment of the implementation of IVDR requirements based on the new MDCG guidelines.
- We also support you in the area of clinical affairs in the approval process for your clinical studies: e.g. applications to the ethics committee and the higher federal authority (BOB).
Revision and adjustment of the quality management system:
- analysis of the need for changes,
- adjustment of processes,
- assistance with audits.
Other services:
- software development process according to IEC 62304,
- performance evaluation for software products,
- support for the documentation and the QMS for in-house in-vitro diagnostics.
Please contact us to learn more about how we can help you with your IVD.
Your point of contact for all your IVD compliance needs



Dunja Schildge-Reichmann
Head of Regulatory Affairs & Quality Management
dunja.schildge-reichmann@metecon.de
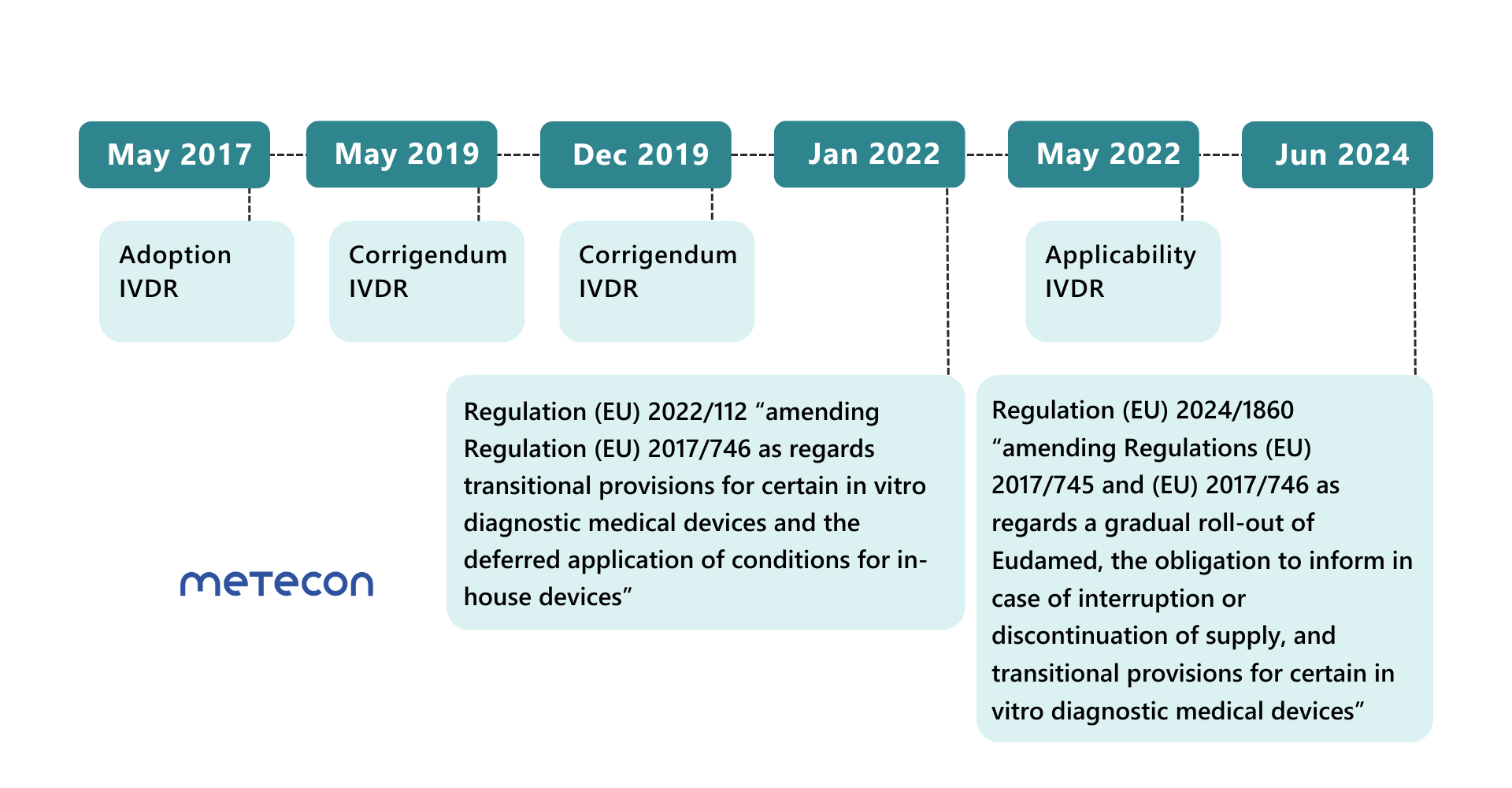
Amending Regulation (EU) 2022/112: First extension of the transition periods for IVD
The transition periods for most product classes were extended at the beginning of 2022. However, the date of application of the IVDR was not postponed. Since May 26, 2022, some requirements of the IVDR must already be met for all product classes, including- post-market surveillance,
- market surveillance (by the relevant authorities),
- vigilance; and
- registration of economic operators and products.
Got any questions? We'd be delighted to help!
Amending Regulation (EU) 2024/1860: Further extension of the transition periods
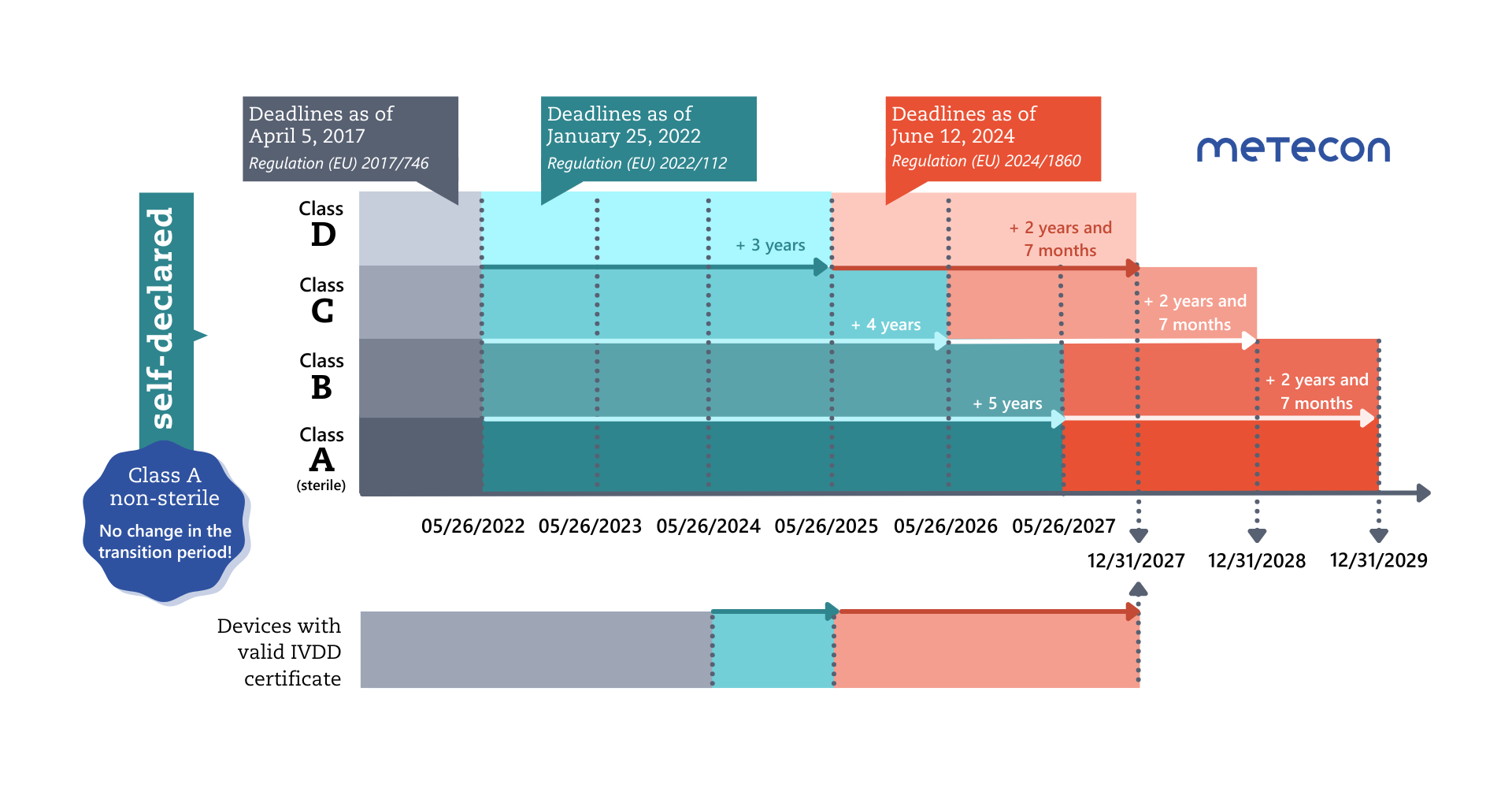
Less than a year before the end of the extended transitional periods for Class D products on May 26, 2025, the EU Commission is publishing a further regulation to extend the transitional periods for IVDs again.These transitional periods can only apply if manufacturers meet certain conditions:
- The conditions from the first regulation extending the transitional periods ((EU) 2022/112) remain in place: The devices must continue to comply with the IVDD and there must be no significant changes to their design and intended purpose.
- the devices do not present an unacceptable risk to the health or safety of patients, users, or other persons or to other aspects of public health protection. Note: this is the responsibility and task of the competent authorities (Articles 89 and 90 of the IVDR) and does not constitute an additional requirement for manufacturers;
- the manufacturer has established a quality management system (QMS) in accordance with Article 10(8) by May 26, 2025, at the latest;
- for legacy devices, an application for the IVDR conformity assessment procedure must be submitted by the manufacturer to a notified body by a specific date: by May 26, 2025 (class D), May 26, 2026 (class C), and May 26, 2027 (class B and class A sterile devices);
- a written agreement with the Notified Body must be submitted within a further 4 months of the respective deadline;
This means that only those legacy devices can benefit from the new transitional rules that are actually to be transferred to the IVDR, or legacy devices for which a corresponding replacement product is to be approved (Art. 3c (e)).
Want to bring clarity to your products? We are always available for a conversation.
Validity of existing certificates
The following applies to the renewal of existing certificates according to the amended Article 110 of the IVDR:"Certificates issued by notified bodies in accordance with Directive 98/79/EC from 25 May 2017 that were still valid on 26 May 2022 and that have not been withdrawn thereafter shall continue to remain valid after the end of the period indicated on the certificate until 31 December 2027.Certificates issued by notified bodies in accordance with that Directive from 25 May 2017 that were still valid on 26 May 2022 and that have expired before 9 July 2024 shall be considered to be valid until 31 December 2027 only if one of the following conditions is fulfilled:(a) before the date of expiry of the certificate, the manufacturer and a notified body have signed a written agreement in accordance with Section 4.3, second subparagraph, of Annex VII to this Regulation for the conformity assessment in respect of the device covered by the expired certificate or in respect of a device intended to substitute that device;(b) a competent authority of a Member State has granted a derogation from the applicable conformity assessment procedure in accordance with Article 54(1) of this Regulation or has required the manufacturer, in accordance with Article 92(1) of this Regulation, to carry out the applicable conformity assessment procedure."
Technical documentation for in vitro diagnostics
The technical documentation must be revised and adapted in the entire context of the IVDR for all existing products. Risk management results influence the performance evaluation and usability of your IVD and vice versa. The purpose, requirements, specifications, verification/validation, and labeling must be coordinated. Traceability is therefore required from the product idea to the finished product.Post-market surveillance (PMS) requirements also need to be implemented. Depending on the risk class, different reports are required: PMS reports for class A & B or the Periodic Safety Update Report (PSUR) for class C & D. Not to be forgotten: a summary report on safety and performance (Summary of Safety and Performance, SSP) for Class C & D products is also required.Information on the content of the required reports, which are always preceded by a plan, can be found in the IVDR and in specific guidelines from the MDCG (Medical Device Coordination Group).
How much time does iVDR adaptation cost? A realistic calculation including an example
From our discussion with manufacturers, we know that 200 working hours are often calculated for the revision of a single product file. So far, so good: That means a pure, dedicated working time of about 5 weeks for a full-time employee. Why "pure" working time? Because this calculation would require the following:- The full-time employee has been trained;
- a project plan for the exact procedure should be ready;
- the full-time employee knows exactly how to implement the requirements;
- there are no other tasks for your regulatory affairs employees within these 5 weeks;
- cooperation between departments is smooth and without incident.
Is this scenario actually realistic? Our experience shows that this rarely works. In your planning, you should also consider that your quality management processes will also have to be revised and new templates should be created.
Need support? We look forward to contributing to your team's success.
Your benefits when working with us
- We are your reliable partner for adapting your QM processes and developing and revising the technical documentation of your IVD.
- You will receive all documents for your technical documentation ready for signature on your templates for approval (international) or product certification (EU). We would also be happy to provide you with templates and advise you on choosing the right format for your technical documentation (IMDRF, STED).
- Our 20 years of experience in the documentation, the certification process, and also in the verification and validation of IVDs ensure that we achieve your goals quickly and efficiently.
- Our GAP analyses provide recommendations for action at all levels of your regulatory activities (QM system, technical documentation, product portfolio).