The technical documentation demonstrates the conformity of your in vitro diagnostic device with the European regulation, the IVDR. In Annex II of the EU Regulation 2017/746 on in vitro diagnostics (IVDR), these requirements are divided into six chapters, plus the requirements from Annex III on post-market surveillance. The requirements are comprehensive compared to the previous requirements of the IVDD.The extended regulation of responsibilities is also new: In addition to manufacturers, the most important stakeholders also include importers and distributors.The contents of the Technical Documentation (TD) must be compiled and required, missing performance data, for verification and/or validation of your product must be obtained. For legacy devices (existing products), the effort depends on the quality and completeness of the data already available. Therefore, one of the first steps in preparing your product for European market authorization is to evaluate the available data.
The IVD Regulation IVDR holds many innovations in store for medical device manufacturers. Our free template "Technical Documentation under the IVDR" supports you in adapting your product file.We now provide you with the IVDR innovations free of charge as a PDF download. Use our IVDR whitepaper "Technical Documentation under the IVDR" as a template and checklist for your IVDR adaptation!
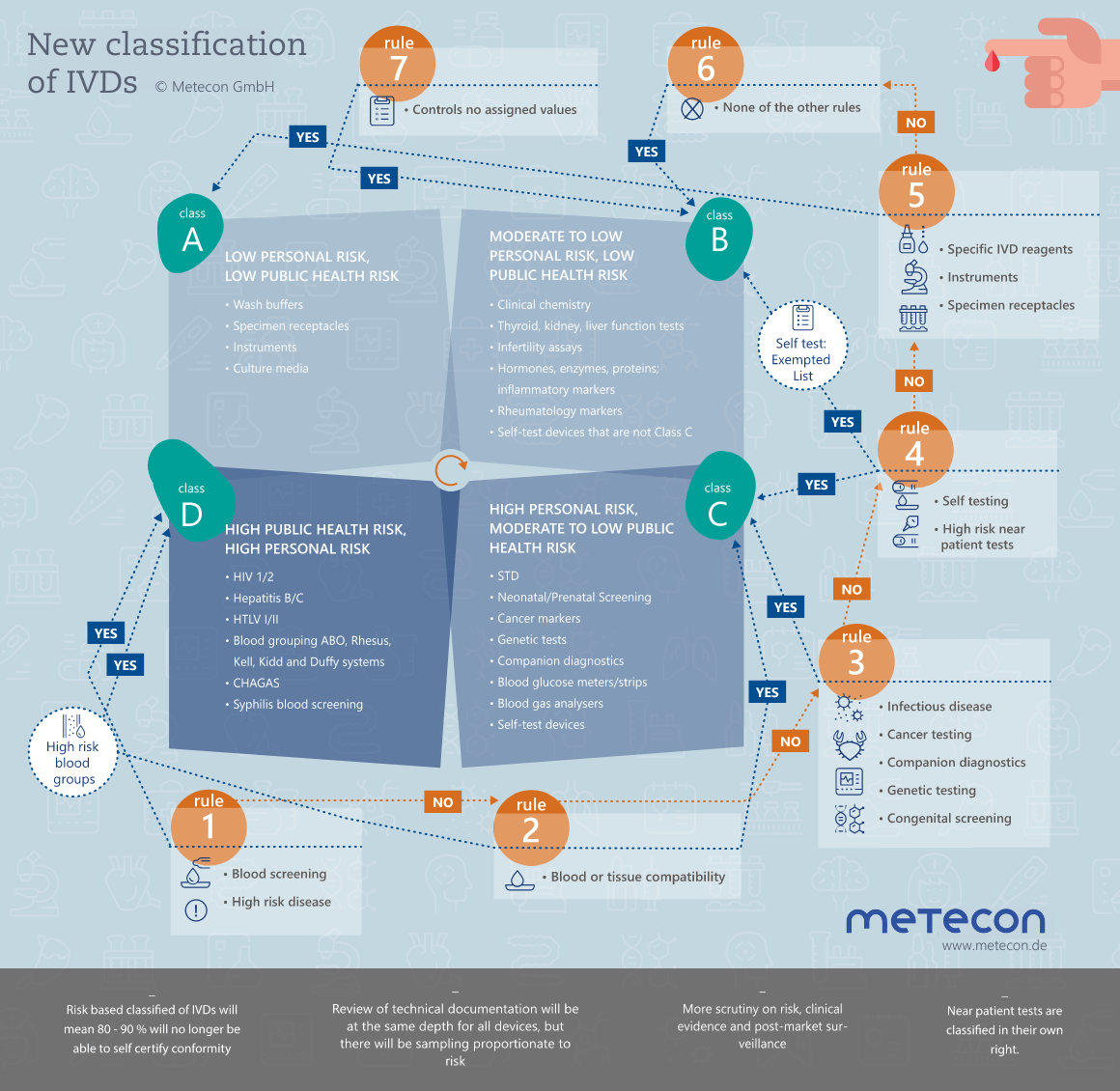
Classification of IVDs
Under the IVD Directive, products are classified according to Annex II as List A or B products, or as products for self-testing and others. New with the introduction of the IVDR regulation is a classification based on seven classification rules into classes A, B, C or D. Depending on the classification of the IVD, different requirements for the conformity assessment procedure and the involvement of the notified body must be observed.
More about Classification of IVDs
The benefits of working with us
- We are your reliable partner for adapting your QM processes and developing and revising the technical documentation of your IVDs.
- You receive all documents for your technical documentation based on your templates and ready to be signed for approval (international) or product certification (EU).
- Our experience with technical documentation and the certification process as well as with verification and validation ensures that we reach your goals quickly and efficiently.
- Our GAP analyses deliver recommendations on all levels of your regulatory activities (QM system, technical documentation, product portfolio).
- Benefit from our network. We support you in the search for a notified body, suitable laboratories for analytical tests, in identifying sample suppliers, in submitting applications for clinical studies (BfArM, ethics committee), in the search for a suitable CRO or a usability laboratory.
We also offer you advice and support with:
- Risk management process according to ISO 14971,,
- Usability according to ISO 62366,
- Software development process according to IEC 62304,
- Clinical performance studies according to ISO 20916,
- Verification and validation of your products.

Dunja Schildge-Reichmann
Head of Regulatory Affairs & Quality Management
dunja.schildge-reichmann@metecon.de
IVD classification: Where it all begins
Have you already classified your products according to the new classification rules of the IVDR, Annex VIII, into classes A, B, C, or D? Definitely not always easy, and therefore we advise you to consult the "MDCG 2020-16 Rev.2 - Guidance on Classification Rules for in vitro diagnostics under Regulation (EU) 2017/746" of February 2023 if needed.Of course, the first step is to be sure that the devices in question are in vitro diagnostic devices. It is not always easy to distinguish between a medical device and an IVD, or between an IVD and a laboratory accessory. The "Manual on borderline and classification for medical devices under Regulation (EU) 2017/745 on medical devices and Regulation (EU) 2017/746 on in vitro diagnostics" is helpful. Please make sure you keep the guide up to date as changes are made from time to time.Intended use is a prerequisite for defining or determining your product as an IVD and for its classification. Clarify the most important questions in advance: What can/should my product do? Who is the user? Who is the beneficiary? Are there any limitations? - This sounds easier than it is.Need help classifying your IVD? We look forward to working with you to find a solution for your products.