Depending on the target market, the regulatory requirements for the approval of in vitro diagnostics (IVD) differ. It is relevant whether the markets are in Europe, the UK, Switzerland, the USA, China or the MDSAP (Medical Device Single Audit Program) countries, etc., because different regulations and laws apply in each case. The differences between the regulations are sometimes large and are subject to constant change, which must be taken into account. Manufacturers of IVDs must meet numerous requirements from product development to market launch. If it is clear from the outset that the product is to be marketed in several markets, then the relevant regulations- such as the IVDR for the EU - must be observed right from the start. If an existing market is to be expanded, it must be checked which additional requirements must be observed.
What are the tasks of a PRRC?
The tasks of a PRRC (Person Responsible for Regulatory Compliance) are firmly defined in the IVDR (Article 15) and the MDR. A PRRC is responsible for, among other things:
- Regulatory compliance
- Checking the conformity of products before product release according to QMS
- Preparation and continuous updating of the Technical Documentation and EU Declaration of Conformity
- Post-market surveillance (establishment of a post-market surveillance system)
- Fulfilment of reporting obligations (vigilance)
Prerequisites and requirements for a PRRC
In the EU, Article 15 of the IVDR requires manufacturers of in vitro diagnostic medical devices to have a Person Responsible for Regulatory Compliance (PRRC). The registration of the responsible person takes place in EUDAMED. PRRC must not only fulfil his duties according to IVDR, but also some requirements: At least one year of professional experience in regulatory affairs or quality management AND a degree in a recognized field of study (e.g. law, medicine, pharmacy) OR four years of professional experience in regulatory affairs or quality management related to in vitro diagnostic medical devices.
Development of your international approval strategy in the most effective way
International approval is complicated: Our Regulatory Affairs IVD team is the partner at your side who is well versed in the regulatory requirements for international approval.Together with you, we develop the optimal approval strategy for your products and your target market(s). We provide you with significant support in ensuring that your products are correctly classified and quickly approved in your target markets and, if required, will also be happy to take on associated tasks for you.Schedule a free appointment now to plan your strategy together
The international approval of IVD products is complicated - a strategic approach to regulatory affairs saves time and money.
Our regulatory affairs services for IVDs includes:
Approval strategy
Creation of the entry documents
- Analysis of (inter-)national legal requirements in addition to the preparation and further development of corresponding approval strategies,
- Monitoring of registration procedures, preparation of approval applications for national and international registration procedures, planning and preparation of applications for amendments and renewals,
- Establishing and maintaining contacts with national and international regulatory authorities, registration authorities, notified bodies, and national and international health authorities,
Creation of the entry documents
- Support in the preparation of submission documents for the approval of in vitro diagnostic (IVD) devices in Europe (CE),
- Support in the preparation of the submission documents (Registration Master File) for the approval of in vitro diagnostics (IVD) in various countries such as USA (FDA), Australia (TGA), Canada (CMDR), China (CFDA) or Japan (PMDA) according to the respective legal requirements,
- Research specific regulatory requirements for specified target countries and answer regulatory questions (e.g. UDI, classifications, implementation, etc.)
Do you have a plan yet?
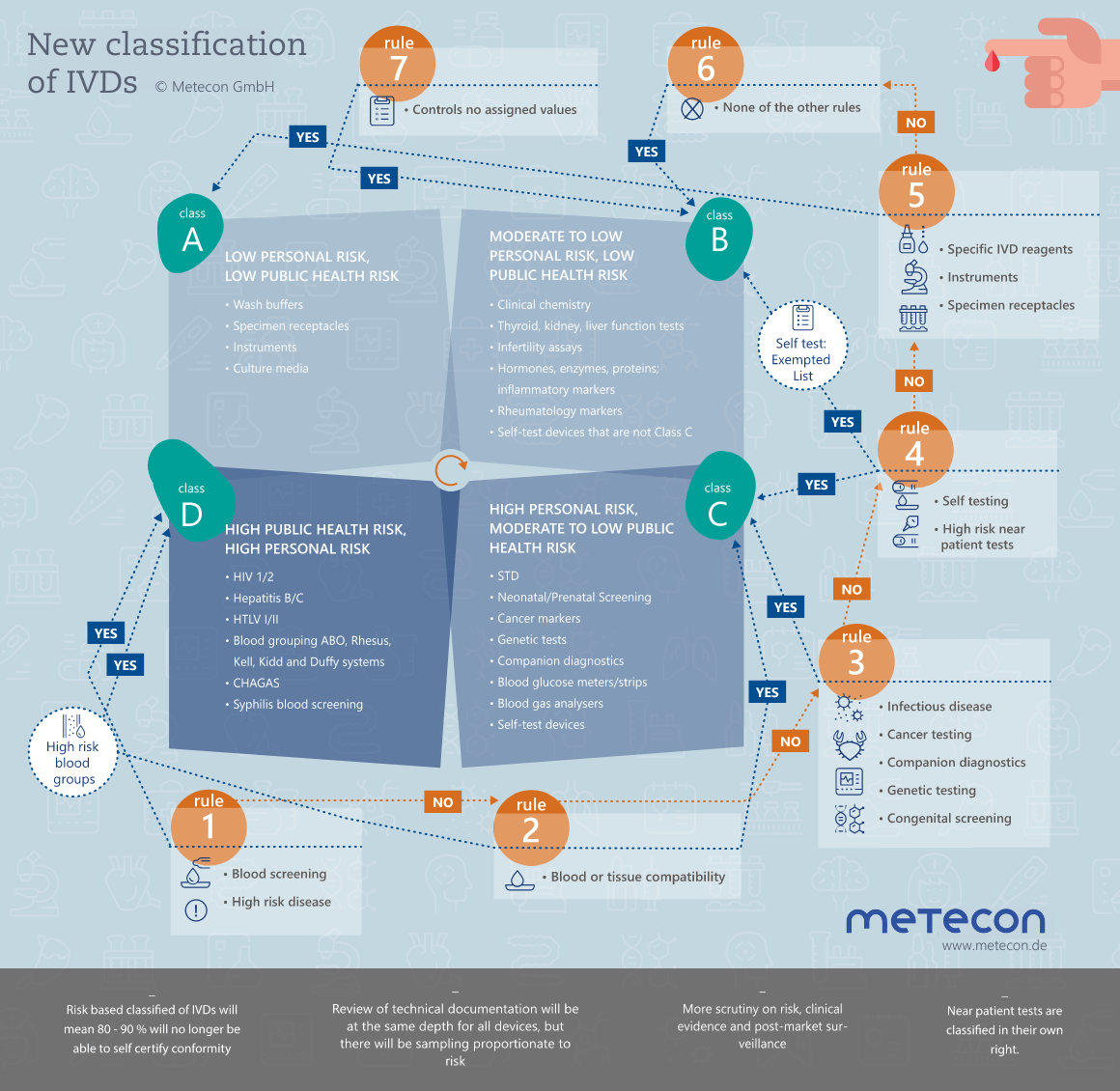
The new classification rules and the associated increased regulation via the Notified Body are confronting manufacturers with extreme challenges. In view of the time constraints, the revision of technical documentation must be well planned and thought through. We would be happy to clarify your needs in an initial, non-binding discussion.Arrange an appointment now and benefit from our know-how!
How may we support you?
Different countries, different customs – that is all the more true in the context of certification and market entry. Use our know-how and stay on the safe side.
International approval
International approval
“A goal without a plan is just a wish” -
Let us develop the ideal strategy for you together at an early stage to ensure your international success.
Approval strategy
Let us develop the ideal strategy for you together at an early stage to ensure your international success.
Approval strategy