Artificial intelligence (AI) and machine learning (ML) are making their way into the world of in vitro diagnostic medical devices (IVDs). While they elevate the performance of IVDs to a new level, they also present manufacturers with new regulatory challenges. For the first time, the European AI Act (Regulation (EU) 2024/1689) introduces binding rules for the use of AI in IVDs, thereby raising the bar for safety in diagnostic systems.Yes, the regulatory burden continues to grow. But this regulation is justified and, if implemented successfully, can put you in a leading position: As a manufacturer of IVDs with AI components, you gain not only regulatory certainty but also market trust. You ensure that your AI-enabled IVD complies with all documentation requirements and demonstrate that it is secure against tampering, data loss, and malfunction (keyword: cybersecurity), throughout its entire life cycle.Planning an IVD with AI functionality? Get an overview in a no-obligation consultation with our experts. Contact us now!
Our services for your IVD
Developing a regulatory strategy:
Partner with Metecon to receive the support you need: from a thorough analysis of the regulatory foundations for your AI/ML-enabled IVD to the development of a tailored market access strategy aligned with applicable European and international regulations.Analyzing and interpreting requirements:
We identify and prioritize the relevant legal, normative, and regulatory requirements on your behalf, including the AI Act, the IVDR, ISO/IEC standards, and country-specific regulations. This may include ISO 13485, ISO/IEC 42001, ISO/IEC 23894, ISO 14971, IEC 62304, IEC 81001-5-1, IEC 82304-1, IEC 60601-1, ISO/IEC 27001, ISO/IEC 27701, IEC TR 80002-1, ISO/IEC 24029, ISO/IEC 23894, and FDA guidances.Achieving clarity through gap analysis:
We identify existing gaps in your processes and documentation and point out missing information, evidence, or structural elements to ensure full regulatory compliance and audit readiness.Creating and optimizing the technical documentation:
We prepare and supplement your documentation to ensure compliance and verifiability, from performance evaluation and risk management to software-related evidence.Adapting processes for AI/ML:
We tailor your internal workflows and quality management systems so that AI-specific requirements can be efficiently addressed and sustainably implemented.Implementing AI data management:
Together, we establish a structured data management system that fully meets the requirements for training, tuning, and testing data.Comprehensive 360° support through your market access process:
We provide end-to-end support throughout your regulatory process, from regulatory assessments to audit preparation and support.AI Act mapping and use case analysis:
How is your specific use case classified under the AI Act? Which articles and requirements apply, and what actions follow? We analyze this for you.Securing and monitoring AI systems:
Together with you, we develop transparent concepts for safety and human oversight, from system architecture to deployment.Evaluating training, tuning, and testing data:
We systematically analyze your data sources regarding their origin, suitability, and compliance with regulatory requirements, helping you meet traceability obligations.Communication with your notified body:
We prepare you for interactions with notified bodies (EU) or regulatory authorities (e.g., U.S. FDA) and provide expert support for AI-specific questions.Workshops and in-house training:
Take advantage of our practical expertise on the regulatory implementation of AI systems—customized to your teams’ experience and responsibilities.
Let’s get together and assess how you can successfully navigate the IVDR and the AI Act. Schedule your free initial consultation now!
What does the AI Act mean for manufacturers of IVD?
The AI Act requires all providers of AI systems in the EU to meet extensive transparency, safety, and documentation obligations. In vitro diagnostic devices (IVDs) with AI components—such as those used in image analysis, algorithm-based risk assessment, or automated data interpretation—are almost certainly classified as high-risk AI systems.This high-risk classification brings with it a number of additional requirements, including the need for a detailed risk analysis specific to the AI component, implementation of technical and organizational human oversight measures, and documentation of training, tuning, and testing data. Moreover, the entire system architecture must ensure transparency, robustness, and traceability.These requirements apply in addition to the IVDR, not instead of it. Manufacturers must therefore address two complex regulatory frameworks and ensure they are well integrated.The earlier you begin addressing the requirements, the smoother your CE marking process will be.
Key points to be prepared for:
- Documentation of training, test, and validation data
- Demonstration of robustness, accuracy, and control mechanisms
- Transparent decision-making processes and explainable system logic
- Risk assessment and mitigation of adverse effects
- Integration of AI components into existing IVDR-compliant documentation
Navigating dual compliance: How the IVDR and AI Act work together
The AI Act does not replace existing regulations such as the IVDR—it complements them. This creates new obligations and requires a deliberate approach to ensure both sets of requirements are aligned as effectively as possible.
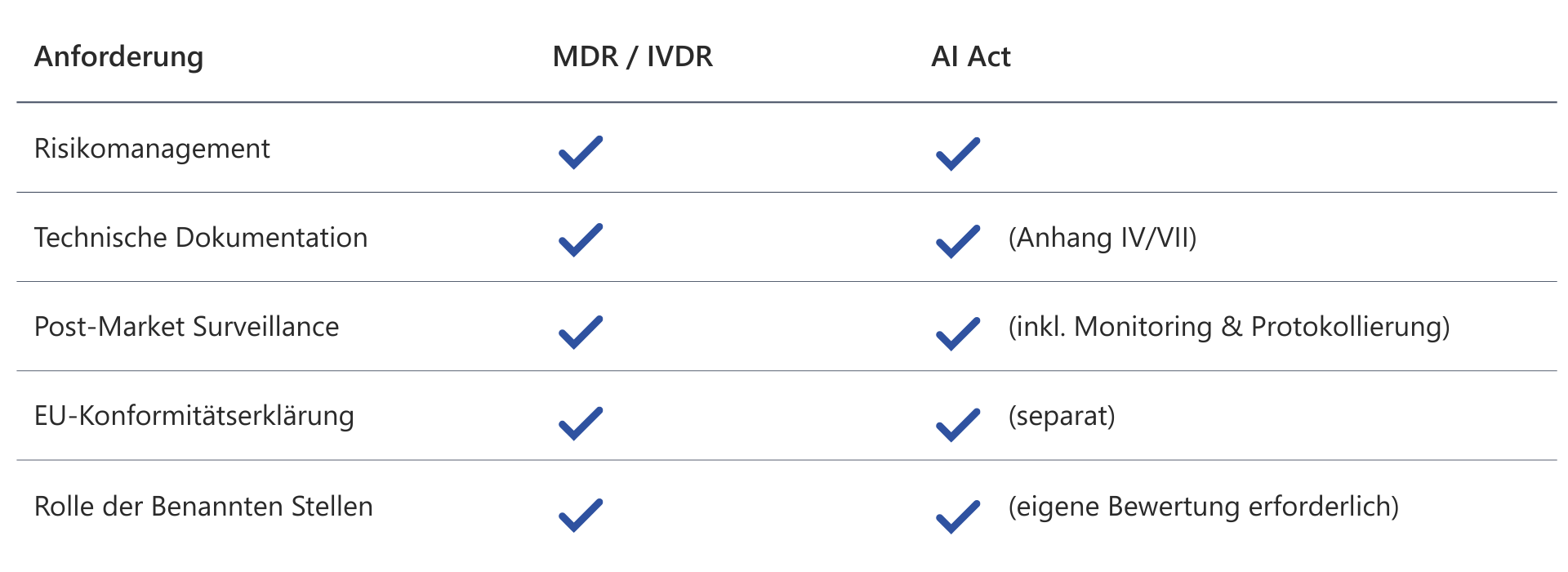
AI Act: Key terms and concepts explained
The AI Act applies to products placed on the market in the EU and to AI systems that affect individuals within the EU.
Transition periods under the AI Act: What manufacturers of IVD need to know
The AI Act has been in force since February 2025. For manufacturers of IVDs with AI components, this marks the start of a transition period in which strategic decisions are critical. Beginning in 2027, the new requirements will be enforced.Our recommendation: Assess early on whether your products fall under the AI Act and develop a clear implementation strategy—so that market access is not delayed.

Cybersecurity and the AI Act: Essential for AI-based IVDs
Cybersecurity is not just a technical requirement - it is a regulatory obligation. AI-powered IVD systems must be designed to remain secure against tampering, data loss, and malfunction throughout the product’s entire life cycle.
Security requirements for AI in IVD systems:
- Protection of patient and input data
- Traceable decision logic and audit trails
- Logging and monitoring of system activities
- Early-warning mechanisms for adverse effects
- Security strategy integrated into the quality management system
The position paper "European Artificial Intelligence Act" published by Team-NB in April 2025 makes one thing very clear: cybersecurity for AI in medical devices goes far beyond traditional IT security. The system architecture must be both technically robust and demonstrably compliant with regulations from day one.We show you how to effectively combine cybersecurity and regulatory requirements to ensure your AI-enabled IVD can gain market approval with confidence.Stay in control of cybersecurity from the day one and get expert support for your AI-based IVD now. We look forward to meeting you!
AI Act for IVD: Acting now ensures your future flexibility
The AI Act is already a reality. The transition periods have begun, and many of its provisions overlap with existing requirements under the IVDR and international standards. For IVD manufacturers with AI features, this is a wake-up call: late action risks delays, resource bottlenecks, and high correction costs.Now is the time for a thorough assessment - for three compelling reasons:1. Corrections are costly, prevention pays off
Reacting only once notified bodies or regulators raise concerns often leads to expensive rework. Early evaluation of your AI components under the AI Act saves time and reduces regulatory risks.2. Product development requires regulatory clarity
AI features are often planned technically before being assessed from a regulatory perspective. Early classification avoids detours in development, documentation, and risk analysis.3. Regulatory foresight builds trust
Companies that actively integrate the AI Act into their development strategies demonstrate responsibility to authorities, partners, and users. This not only facilitates smoother approvals but also long-term market acceptance.We help you achieve regulatory compliance and build a strategy that stands the test of time.Schedule your orientation session now - non-binding, but essential for your success.