The term medical software encompasses a wide range of products used in therapeutic or diagnostic contexts – and its importance is growing rapidly in light of the ongoing digital transformation in healthcare. This includes software for medical devices (with a strong focus on artificial intelligence (AI), cybersecurity, and the AI Act), software embedded in medical devices – also referred to as embedded software – and software that qualifies as a medical device, known as stand-alone software.Manufacturers of medical devices and in vitro diagnostics are faced with the challenge to develop cost-efficient products of high quality that comply with the regulations. Both MDR and IVDR require manufacturers to ensure their devices' safety and the expectations of software documentation are high. Especially small and medium-sized businesses often experience a lack of resources and insufficient knowledge of general regulatory requirements. As your holistic service provider, we guide you through all stages of your devices’ software lifecycle – competently and reliably.Contact us to find out more
... and what does “holistic” mean?
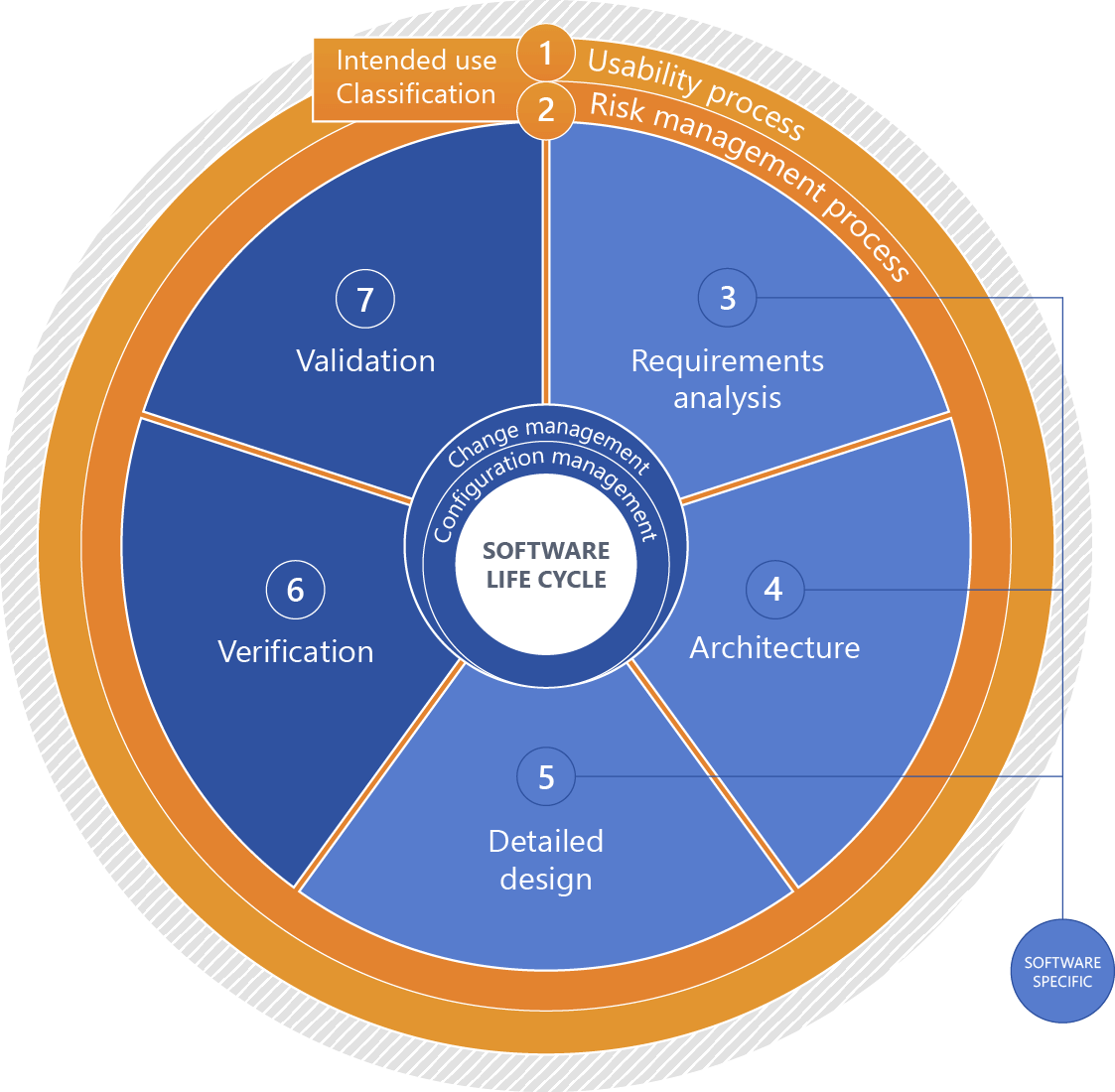
The software development process is a highly networked process. We align it with all processes along the development chain; for instance risk management (ISO 14971) just as usability (IEC 62366) are taken into account.Please click for an explanation of the figure:
+1 - Intended use
Intended use of the medical device.
+2 - Classification (software safety classification)
Software classification based on the risks associated with the software considering measures of risk governance implemented outside the software with a focus on expectable damage.
+3 - Requirements analysis
Systematically deriving the software requirements from the system requirements. For standalone software, the software requirements are identical to the system requirements.
+4 - Architecture
Organizational structure of a system or a component (EN 62034, 3.3).
+5 - Detailed design
Detailed continuation of the system architecture to a specific solution for implementation.
+6 - Verification
Verifying compliance with defined requirements by providing objective proof (EN 62304, 3.33).
+7 - Validation
Validating compliance with the requirements for a specific intended use by providing objective proof (EN 82304-1, 3.23).
Regulatory requirements of medical software
Safety requirements
Safety: Protection against device malfunctions due to software failures,Security: Protection of the software against hacker attacks and attacks from outside,Privacy: Compliance with the requirements in terms of data privacy.
The regulatory requirements are derived from the medical device regulation (MDR). The general safety and performance requirements define the need for establishing a software lifecycle (refer to MDR Annex I, Chapter I, 17.4). The software lifecycle is sufficiently represented in EN 62304.EN 60601-1 and 82304 represent an umbrella for EN 62304 as EN 62304 alone does not represent the medical device level. Therefore, IEC 62304 for instance does not include requirements for validating a medical device These requirements are defined in IEC 60601-1 for software as a component of medical devices and in IEC 82304 for software as a medical device.The standards mentioned actually reflect the current state of the art but merely EN 60601-1 is included in the Official Journal of the European Union as a harmonized standard in its current version (EN 60601-1:2006/A1:2013). Neither the current version of EN 62304 (EN 62304:2006/AC:2008/A1:2015) nor the current version of EN 82304 has been published in the Official Journal of the European Union as a harmonized standard. In April 2018, the first part of EN 82304 was published (EN 82304-1:2018).It is recommended to consult with your notified body before applying non-harmonized standards. And of course, none of the standards is harmonized with respect to the MDR. Again, you will have to consult your notified body regarding the application.Do you have any questions? We are happy to answer them - contact us!
We are happy to support you in the following topics:
Software may be an individual medical device enabling medical decisions.
Software as a Medical Device (SaMD)
Software as a Medical Device (SaMD)
Software may also be part of a whole, for example as firmware in almost any complex medical device.
Software in a Medical Device
Software in a Medical Device
Software is increasingly becoming a strategic success factor – especially in the context of AI, cybersecurity, and regulations like the AI Act.
Software for Medical Devices
Software for Medical Devices