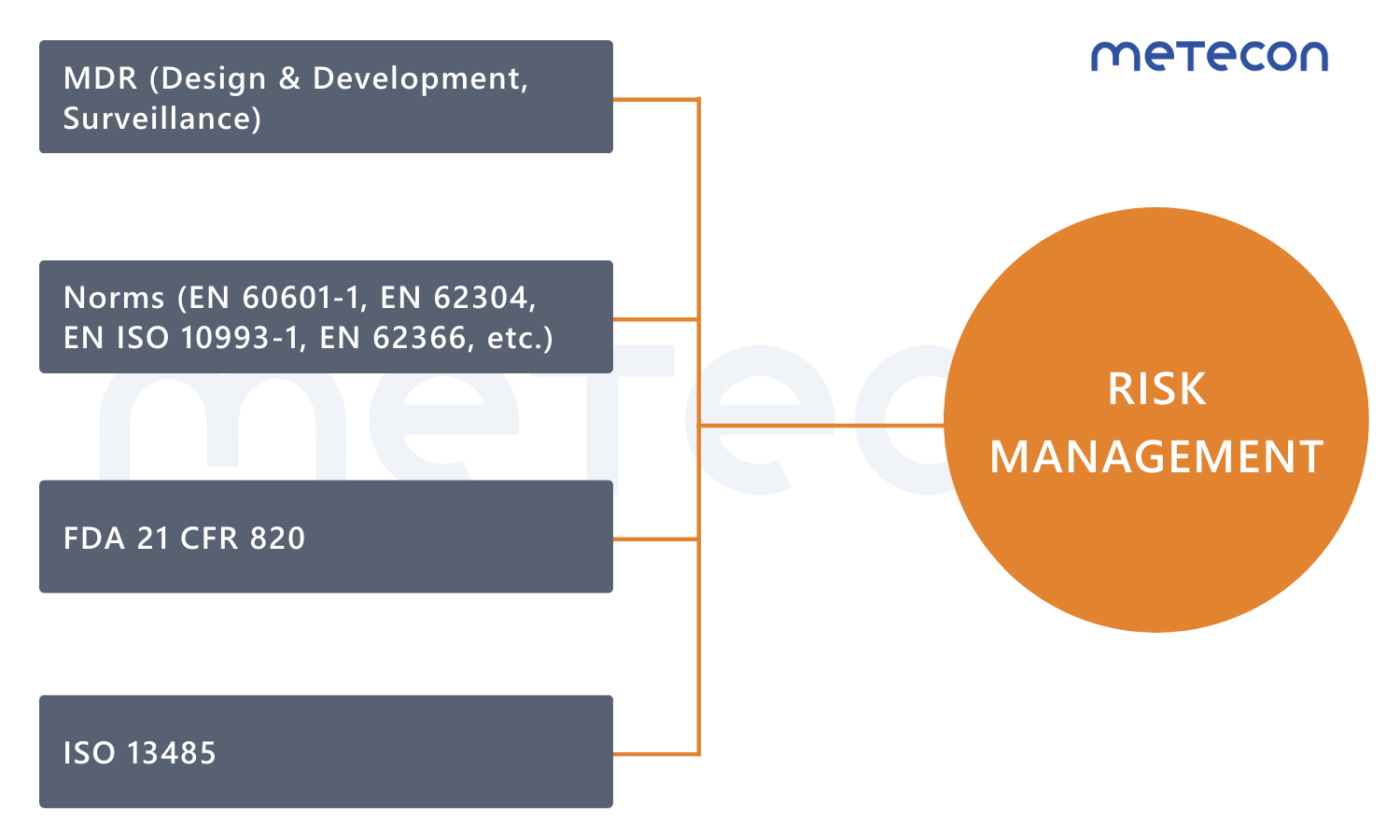
Effective risk management is essential for regulatory compliance of medical devices. In accordance with EN ISO 14971, the requirements of the Medical Device Regulation (MDR), as well as relevant standards (e.g., EN 60601-1, EN ISO 10993-1, EN 62304, EN 62366), manufacturers must systematically identify, analyze, evaluate, monitor, and control risks.Why risk management?
Effective risk management protects patients, users, and the environment from potential hazards while ensuring the performance of your medical device.When does risk management start and how long does it last?
Ideally, risk management starts in the early development phase and extends throughout the entire product lifecycle.How to achieve effective risk management?
By conducting structured risk analyses, identifying and assessing hazards, and defining and verifying risk mitigation measures through an interdisciplinary team.We work hand in hand with your team to develop customized risk management solutions.
Learn how we can efficiently support your team – contact us today!
Effective risk management protects patients, users, and the environment from potential hazards while ensuring the performance of your medical device.When does risk management start and how long does it last?
Ideally, risk management starts in the early development phase and extends throughout the entire product lifecycle.How to achieve effective risk management?
By conducting structured risk analyses, identifying and assessing hazards, and defining and verifying risk mitigation measures through an interdisciplinary team.We work hand in hand with your team to develop customized risk management solutions.
Learn how we can efficiently support your team – contact us today!
Our risk management services
- Gap Analysis & Compliance Check: Identification of regulatory gaps, e.g. in the risk management file
- Documentation & Process Integration: Development and optimization of the risk management process, as well as integration of risk management results into the technical documentation: creation and maintenance of the risk management plan, risk analysis, and risk management report, which includes ensuring traceability to verification & validation, usability, post-market surveillance, and other relevant components of the technical documentation
- Workshops & Training: Customized training sessions with your team on EN ISO 14971 and best practices
- Hands-on Support: Direct collaboration with your team to implement regulatory requirements, including full creation and review of submission-ready documents based on your defined processes
- Rapid Assistance for Non-Conformities: Reliable support in addressing non-conformities to ensure market approval
Whether it’s post-market surveillance (PMS), usability, or biological safety – our interdisciplinary team of regulatory experts provides the knowledge and experience needed for a safe and efficient risk assessment.
Let's optimize your risk management processes together – safe, compliant, and sustainable!
EN ISO 14971: Applying risk management to medical devices
Both the Medical Device Regulation and various harmonized standards require a structured risk management process. EN ISO 14971 defines the fundamental principles and requirements for the risk management of medical devices.The standard provides a framework that enables manufacturers to identify, assess, and control risks associated with their medical devices.
It is the one that is most closely linked to other standards. Give EN ISO 14971 the attention it deserves!
Risk management requires interfaces with various processes
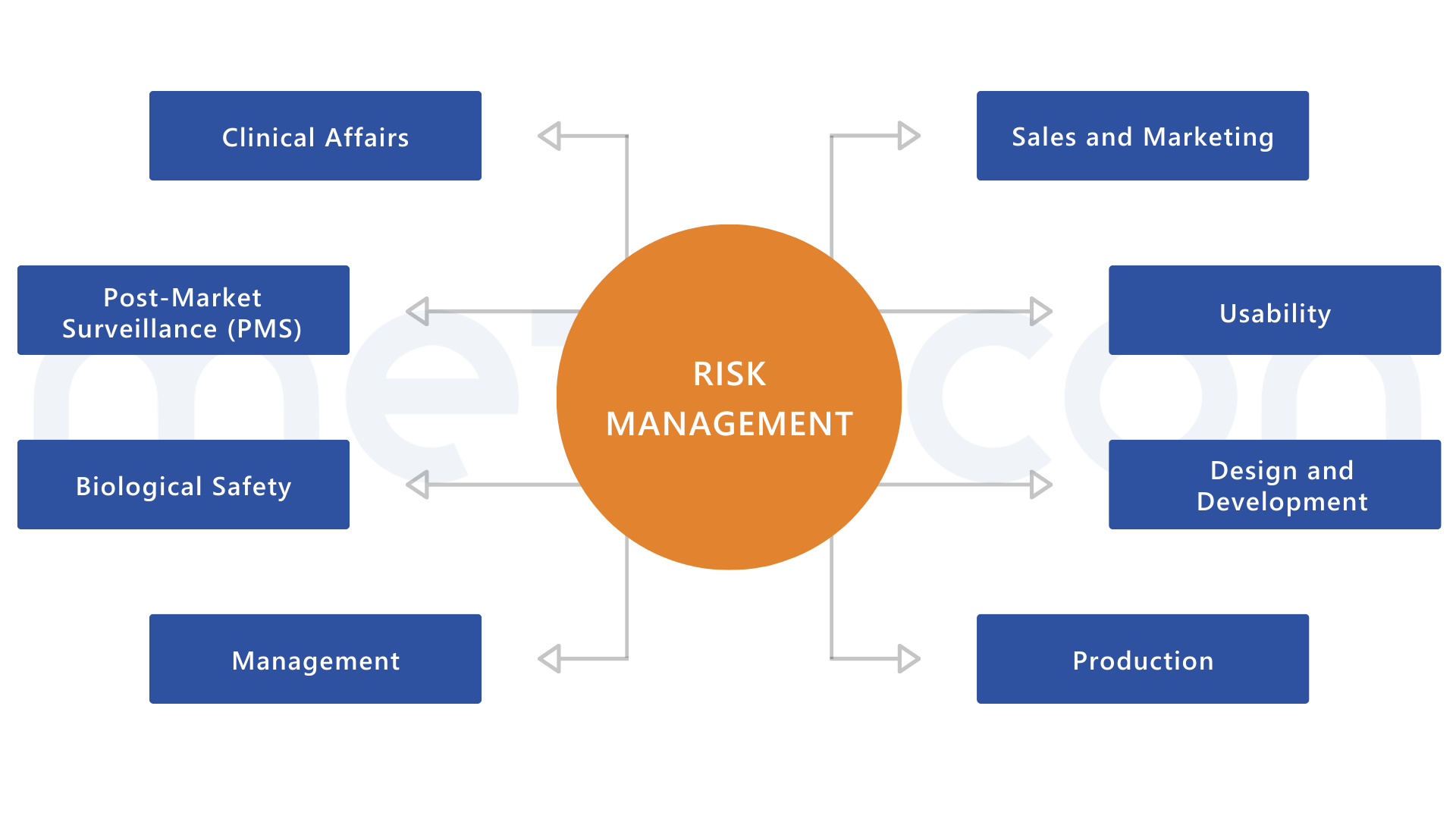
Regulations closely linked to EN ISO 14971
No other standard is as closely connected to other norms and regulations as EN ISO 14971. Give it the attention it deserves!Unsure if you need support or to what extent? Let’s find out together: contact us for a consultation – no strings attached!