In-house IVDs under the IVDR: Why medical laboratories need to act now
03/06/2025
Do you have any questions about the article or would you like to find out more about our services? We look forward to hearing from you!Make a non-binding enquiry now
With the In Vitro Diagnostic Medical Device Regulation (EU) 2017/746 (IVDR), which came into force on May 26, 2022, in-house in vitro diagnostic medical devices - known as in-house IVDs or laboratory developed tests (LDTs) - have also come under stricter regulatory control. Many medical laboratories in Europe now face the challenge of continuing to operate these products in compliance with standards. What previously went largely unnoticed is now subject to extensive requirements: from risk management and performance evaluation to market availability testing from 2030 onwards. What requirements must medical laboratories already meet today for their in-house products? Which tests can still be manufactured and used as in-house IVDs? In this article, we provide a practical overview of the applicable obligations - and show what laboratories should now pay attention to in order to keep their in-house IVDs compliant and usable in the long term.
Compliance with Annex I is not a new requirement for in-house IVD manufacturers in Germany, as this was already required by the former German medical devices act (Medizinproduktegesetz - MPG) prior to the IVDR. However, this is one of the most complex requirements for manufacturers of in-house IVDs: to comply with the requirements of Annex I, a large amount of evidence and documentation must be produced, similar to that required for the preparation of technical documentation for CE-marked IVDs. In addition, Annex I also includes requirements for product-specific risk management, consideration of usability, and performance evaluation.The two other requirements, namely hat in-house IVDs may not be placed on the market or manufactured on an industrial scale, as well as the requirement for a suitable quality management system (QMS - see next paragraph), are not new, but were also previously required by the MPG.
Please note: Many laboratories already work in accordance with EN ISO 15189, which, however, does not cover requirements for development and manufacturing. For these aspects, it is recommended to supplement this with relevant chapters of ISO 13485. Further guidance is provided by MDCG Guidance 2023-1 on the application of Article 5(5) and, in addition, the new ISO 5649:2024 on the design and use of laboratory-developed tests (LDTs). Even though the latter is not legally binding, it can provide valuable practical guidance.Important for Class D products: The requirement for detailed documentation regarding the manufacture, design, and performance of Class D products raises the level of technical documentation for in-house high-risk IVDs even further toward CE-marked IVDs.
To meet this requirement, it is necessary to research similar CE-marked products available on the market.The EUDAMED database is one option that could be used for such research. As an in-house IVD manufacturer or laboratory, you should make good use of the time remaining until the 2030 deadline to develop suitable strategies for continuing your in-house products, as this requirement poses a real challenge. It could mean that laboratories will no longer be able to use many of their in-house products after December 2030.Possible scenarios:
Due primarily to the last point, there is a risk that many in-house IVDs will no longer be permitted to be manufactured and used in the future. In addition, many manufacturers of in-house IVDs may shy away from the high documentation requirements, especially for complex products or products with risk class D, with the consequence that these will then no longer be manufactured and available for use.National differences
The IVDR grants member states the right to impose further requirements on in-house IVDs through national legislation, and the manufacture and use of certain types of in-house IVDs may also be restricted. Individual EU member states may, for example, extend the requirement for detailed documentation to product classes A, B, and C (see also MPDG §88 (1) 10). However, in Germany, for example, no corresponding regulation imposing additional requirements or restrictions on the IVDR has yet been adopted.Please note: In Switzerland, which essentially refers to the IVDR in its own Ordinance on In Vitro Diagnostic Medical Devices (IvDO, SR 812.219) with regard to in-house IVDs, detailed documentation in accordance with Art. 5 (5)g, IVDR is required for all classes A to D (Article 9, IvDO).
 Dr. Sandra Reuter
Dr. Sandra Reuter
What are in-house IVDs - and when are IVDs considered as such?
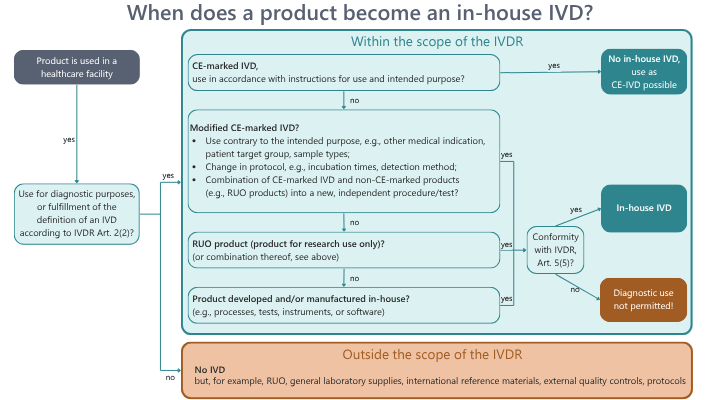
Many medical laboratories use CE-marked products marketed by IVD manufacturers on the one hand, but also in-house IVDs that are manufactured in-house and are not CE-marked to examine patient samples on the other.There is still a great deal of confusion about which products are actually considered as in-house IVDs and which are not. Our short guide will help you get an initial overview.The first question is whether the product has a diagnostic purpose (or is used for diagnostic purposes) and meets the definition of an IVD according to Art. 2 (2) IVDR.General laboratory supplies, international reference materials, external quality controls, and protocols, for example, do not fall under this definition and are therefore not covered by the IVDR.Products for research purposes (research use only, RUO) are also not IVDs by definition; they can only be used for research purposes. But be careful, many laboratories still use RUO procedures for diagnostic purposes: this means they fall within the scope of the IVDR and may only be used if the laboratory can demonstrate compliance with Art. 5 (5) of the IVDR for this product. Even for CE-marked IVDs that are used by the laboratory contrary to their intended purpose and instructions for use, or that are recombined with other products, conformity with Art. 5 (5) must be demonstrated for them to be used by the laboratory for diagnostic purposes. Even specially developed and manufactured products can be used for diagnostics - you guessed it - if they meet the requirements for in-house IVDs.
Which IVDR requirements apply and when?
Regardless of whether you are a laboratory operator who has been using your in-house IVD for years, are currently developing a new product, or have recently developed one: all in-house IVDs used in healthcare facilities such as medical laboratories in the EU must meet the requirements of the IVDR, Article 5 (5). For the first time, in-house IVD manufacturers are subject to fundamentally uniform requirements across all EU member states.Requirements that in-house manufacturers must already meet since May 26, 2022
- Compliance with Annex I of the IVDR: General Safety and Performance Requirements (GSPR).
- Products may not be supplied to other legal entities.
- Products may not be manufactured on an industrial scale.
Compliance with Annex I is not a new requirement for in-house IVD manufacturers in Germany, as this was already required by the former German medical devices act (Medizinproduktegesetz - MPG) prior to the IVDR. However, this is one of the most complex requirements for manufacturers of in-house IVDs: to comply with the requirements of Annex I, a large amount of evidence and documentation must be produced, similar to that required for the preparation of technical documentation for CE-marked IVDs. In addition, Annex I also includes requirements for product-specific risk management, consideration of usability, and performance evaluation.The two other requirements, namely hat in-house IVDs may not be placed on the market or manufactured on an industrial scale, as well as the requirement for a suitable quality management system (QMS - see next paragraph), are not new, but were also previously required by the MPG.
Requirements that in-house manufacturers must meet since May 26, 2024
Due to "Regulation (EU) 2022/112 […] of 25 January 2022 amending Regulation (EU) 2017/746 as regards transitional provisions for certain in vitro diagnostic medical devices and the deferred application of conditions for in-house devices," , the following requirements must be met by in-house IVD manufacturers only from May 26, 2024:- Establishment of a QMS suitable for the manufacture and use (of in-house IVDs)
- Compliance with EN ISO 15189 or, where applicable, national regulations, including national accreditation regulations
- Upon request by the competent authority (in Germany, these are the state-level authorities), provision of information on use, including justification for the manufacture, modification, and use of the in-house IVD.
- Drafting of a publicly accessible declaration of compliance with Annex I
- Detailed documentation on the manufacture, design, and performance data of Class D products so that the competent authority can verify compliance with Annex I
- Ensuring that all Class D products are manufactured in accordance with the above documentation
- Product surveillance: reviewing experience gained from the clinical use of the products and taking the necessary corrective measures.
Please note: Many laboratories already work in accordance with EN ISO 15189, which, however, does not cover requirements for development and manufacturing. For these aspects, it is recommended to supplement this with relevant chapters of ISO 13485. Further guidance is provided by MDCG Guidance 2023-1 on the application of Article 5(5) and, in addition, the new ISO 5649:2024 on the design and use of laboratory-developed tests (LDTs). Even though the latter is not legally binding, it can provide valuable practical guidance.Important for Class D products: The requirement for detailed documentation regarding the manufacture, design, and performance of Class D products raises the level of technical documentation for in-house high-risk IVDs even further toward CE-marked IVDs.
Requirements that in-house manufacturers must meet from December 31, 2030
Due to "Regulation (EU) 2024/1860 […] of 13 June 2024 amending Regulations (EU) 2017/745 and (EU) 2017/746 as regards a gradual roll-out of Eudamed, the obligation to inform in case of interruption or discontinuation of supply, and transitional provisions for certain in vitro diagnostic medical devices," the following requirement of Article 5 (5) IVDR must be met by in-house manufacturers only from December 31, 2030:- Justification for why the specific requirements of the target patient group cannot be met or cannot be met at the indicated level of performance, by a similar product available on the market.
To meet this requirement, it is necessary to research similar CE-marked products available on the market.The EUDAMED database is one option that could be used for such research. As an in-house IVD manufacturer or laboratory, you should make good use of the time remaining until the 2030 deadline to develop suitable strategies for continuing your in-house products, as this requirement poses a real challenge. It could mean that laboratories will no longer be able to use many of their in-house products after December 2030.Possible scenarios:
- Is there no equivalent CE-marked product for your in-house IVD?
Then you may continue to use your in-house IVD. - Is there an equivalent CE-marked product?
- If you can demonstrate that the performance of your in-house product is better, you may continue to use it.
- If the performance of your in-house product is equal to or worse than that of the CE-IVD, you may no longer use your in-house product after December 31, 2030, unless you improve and/or expand the performance of your in-house IVD compared to comparable products, then you may continue to use it after December 31, 2030. Alternatively, you become the manufacturer and turn your in-house IVD into a CE-IVD.
What the IVDR requirements mean for medical laboratories in concrete terms
In summary, the IVDR requirements for the manufacture and use of in-house IVDs mean:- Increased documentation requirements for healthcare facilities. It remains to be seen to what extent member states will extend the requirement for complete, detailed documentation to Class A, B, and C products.
- Increased transparency in the manufacture and use of in-house tests.
- Expanded regulatory oversight activities.
- In-house tests may no longer be used after December 31, 2030, provided that a product with the same intended purpose and performance is available on the market.
Due primarily to the last point, there is a risk that many in-house IVDs will no longer be permitted to be manufactured and used in the future. In addition, many manufacturers of in-house IVDs may shy away from the high documentation requirements, especially for complex products or products with risk class D, with the consequence that these will then no longer be manufactured and available for use.National differences
The IVDR grants member states the right to impose further requirements on in-house IVDs through national legislation, and the manufacture and use of certain types of in-house IVDs may also be restricted. Individual EU member states may, for example, extend the requirement for detailed documentation to product classes A, B, and C (see also MPDG §88 (1) 10). However, in Germany, for example, no corresponding regulation imposing additional requirements or restrictions on the IVDR has yet been adopted.Please note: In Switzerland, which essentially refers to the IVDR in its own Ordinance on In Vitro Diagnostic Medical Devices (IvDO, SR 812.219) with regard to in-house IVDs, detailed documentation in accordance with Art. 5 (5)g, IVDR is required for all classes A to D (Article 9, IvDO).
What medical laboratories should do now
- Expansion or adaptation of your QMS and implementation of missing processes
- Review of your product-specific documentation for completeness and closure of gaps to meet the requirements of Annex I and Art. 5(5) IVDR.
- Preparation of the required justification that there is no similar CE-marked product for your product, so that you may continue to operate it: Conduct product research as quickly as possible and analyze your in-house IVD portfolio to be as well prepared as possible.
Conclusion: New requirements, new uncertainties – act now
The requirements of the IVDR entail significant additional effort for laboratories with in-house tests, both from a technical and strategic perspective. In particular, the deadline at the end of 2030 requires laboratories to take an early look at their own product portfolios. Whether it's quality management, technical documentation, or market analysis, those who create the right structures in good time will be able to overcome the regulatory hurdles and continue to rely on proven in-house IVDs in the future.Would you like to continue using your in-house products safely under the IVDR, or are you unsure whether your laboratory already meets all requirements? There are many ways to achieve compliance — we are happy to help you find the right one.Contact us now for a non-binding initial consultation.
Our blog posts are researched and created with the utmost care, but are only snapshots of the regulations, which are constantly changing. We do not guarantee that older content is still current or meaningful. If you are not sure whether the article you have read on this page still corresponds to the current state of regulation, please contact us: we will quickly place your topic in the current context.

Team Lead IVD
Regulatory Affairs & Technical Documentation


