Change Management in Quality Management - The 5 phases of successful change in medical technology
27/05/2025
Do you have any questions about the article or would you like to find out more about our services? We look forward to hearing from you!Make a non-binding enquiry now
Changes in the medical technology sector are inevitable—whether due to new regulatory requirements, digital transformation, or strategic realignments. Whether you are implementing a RIM system (Regulatory Information Management System) or an electronic quality management system (eQMS), successful change management involves more than just adjusting processes and structures. What truly matters is how well people within the organization are integrated into the transformation process.In "Change Management in Quality Management - Successful transformation in medical technology," we explored the key challenges and success factors in change management. We looked at why acceptance, communication, and corporate culture are just as crucial as a clear strategy and structured processes. Now, the focus shifts to systematically shaping change—with a proven, practical five-phase model.Whether you are introducing a RIM system, an electronic QMS, or undergoing a comprehensive transformation, a structured approach ensures that changes are not only implemented efficiently but also sustainably embedded in your organization.
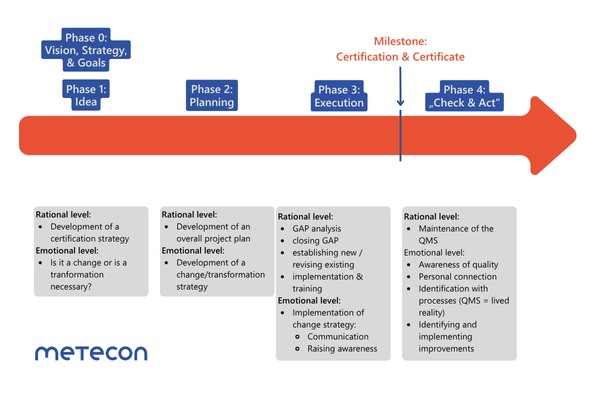
Phase 0 – Vision and strategy
Phase 1 – Idea
Phase 2 – Planning
Phase 3 – Implementation
Phase 4 – Review and adjustment
Stakeholder analysis:
This structured approach enables organizations to successfully implement change—not only in terms of structural execution but also in fostering employee motivation, corporate culture, and long-term acceptance.
Structuring change
A structured change process can be divided into five phases:Phase 0 – Vision and strategy
Phase 1 – Idea
Phase 2 – Planning
Phase 3 – Implementation
Phase 4 – Review and adjustment

Phase 0: Vision, Strategy & Goals
In this phase, a clear vision is developed, from which a strategy with concrete goals is derived:- Where do we want to go?
- What do we want?
- How can we achieve this?
- Company 1 (C1): "We aim to successfully establish our medical device in the European market."
- Company 2 (C2): "Our company operates a QMS according to ISO 9001. To access new markets, we are implementing ISO 13485 and ensuring MDR compliance. We do not only want to supply medical device manufacturers but also manufacture and distribute our own medical devices."
- C1: Transition of the QMS from ISO 13485 & MDD to ISO 13485 & MDR.
- C2: Transition of the QMS from ISO 9001 to ISO 13485 & MDR.
Phase 1: Idea
The nature of the change largely depends on the company’s starting position:- C1: The company is already a medical device manufacturer with a functioning and certified QMS. Its values and culture are aligned with the vision, meaning only selective changes and operational adjustments are necessary. This is referred to as a change process.
- C2: The company is currently a supplier for medical device manufacturers and does not yet operate as a manufacturer itself. The transition to ISO 13485 and MDR requires fundamental structural rethinking. Both structural and organizational adjustments as well as cultural changes are necessary. This is referred to as a transformation process.
Phase 2: Planning
In this phase, the detailed planning of the entire project takes place, including the development of a change or transformation strategy. The following elements must be considered:Stakeholder analysis:
- Who is affected by the change?
- Who has direct or indirect influence on the project's success?
- How do stakeholders differ in terms of risk tolerance, stability needs, and flexibility?
- What specific changes will they experience, and how are they likely to react?
- What are the communication needs of employees?
- Which channels and frequencies are required for effective communication?
- How are criticism and resistance identified and addressed?
- Which conflict resolution strategies are already in place, and which need to be developed?
- How can awareness of new quality standards be strengthened?
- How can potential and opportunities for personal development be highlighted?
- What training and qualification measures are required?
Phase 3: Implementation
The implementation of planned measures requires a comprehensive approach that considers both change dimensions:Rational Level (Project Management):- Conducting a GAP analysis to identify deviations between the actual and target state
- Defining measures to close identified gaps, either by revising existing processes or introducing new ones
- Ensuring compliance with timelines and budgets, along with regular reporting
- Early communication of the project, including its purpose and necessity
- Openly addressing fears and uncertainties
- Recognizing and actively managing resistance
Phase 4: Check and Act
In the final phase, it is assessed whether the implemented measures have achieved the desired success and are sustainably effective. In line with the PLAN-DO-CHECK-ACT principle, this phase involves an active review of both effectiveness and compliance of the revised or newly established processes:Evaluation of effectiveness:- Is the new process effectively and efficiently implemented?
- Are we achieving the desired results?
- Which KPIs support performance measurement?
- Do the new processes meet regulatory requirements?
- Are they being verified through internal system audits conducted by independent auditors?
- Employee discussions and active listening to assess the acceptance of the updated QMS, adjusted processes, and new corporate culture
- Encouraging continuous improvement through open feedback
This structured approach enables organizations to successfully implement change—not only in terms of structural execution but also in fostering employee motivation, corporate culture, and long-term acceptance.
What’s next? Turning your plans into action
Whether you are implementing a RIM system or an electronic QMS, adapting your quality management to new regulatory requirements, or undergoing a comprehensive transformation—successful change follows a structured process.By applying a five-phase approach - from strategic vision to long-term integration - change processes can be effectively managed and successfully embedded within your organization. The key lies in combining methodical planning, strong leadership, and active stakeholder engagement.We provide tailored solutions to support you: from developing an efficient certification and project strategy to change management, stakeholder engagement, and the implementation, optimization, and validation of systems and processes.Every transformation presents challenges—but also significant opportunities. Let’s work together to develop the best strategy for your company. Schedule a free consultation today and take the first step toward successful implementation.Schedule an appointment now!
Our blog posts are researched and created with the utmost care, but are only snapshots of the regulations, which are constantly changing. We do not guarantee that older content is still current or meaningful. If you are not sure whether the article you have read on this page still corresponds to the current state of regulation, please contact us: we will quickly place your topic in the current context.



