State of the Art: How SOTA affects the product life cycle of medical devices and IVDs
10/01/2023
Do you have any questions about the article or would you like to find out more about our services? We look forward to hearing from you!Make a non-binding enquiry now
State of the Art (SOTA) plays an important role in the MDR and IVDR. Within technical documentation (TD), SOTA is strongly linked to various topics, such as clinical evaluation/performance evaluation, scientific validity, risk management, benefit-risk ratio, post-market surveillance (PMS) and post-market clinical follow-up/post-market performance follow-up (PMCF/PMPF).
Our customers’ feedback confirms that SOTA is focused by the notified bodies in the TD reviews. Among other things, they want to know how the creation and updating of SOTA is implemented and whether SOTA is included in the consideration of the benefit-risk ratio. This article explains what this means for you as a manufacturer of medical devices and (in vitro diagnostics (IVDs).
Developed stage of current technical capability and/or accepted clinical practice in regard to products, processes and patient management, based on the relevant consolidated findings of science, technology and experience.
Note: The state-of-the-art embodies what is currently and generally accepted as good practice in technology and medicine. The state-of-the-art does not necessarily imply the most technologically advanced solution. The state-of- the-art described here is sometimes referred to as the "generally acknowledged state-of-the-art"
Furthermore, MEDDEV 2.7/1 rev. 4 (2016) also includes information on what SOTA means in the context of clinical evaluation:The current knowledge/ state of the art in the corresponding medical field, such as applicable standards and guidance documents, information relating to the medical condition managed with the device and its natural course, benchmark devices, other devices and medical alternatives available to the target population.In addition, the "Blue Guide" (Guide for the implementation of the EU product regulations 2016) shows which assumptions are made about SOTA in the context of the presumption of conformity:The concept of essential requirements is based on the assumption that the harmonised standards reflect generally acknowledgeable state of the art and the ESO review standards regularly. [...]Furthermore, even if the manufacturer has not used harmonised standards, a change in the relevant harmonised standard could mean a change in the state of the art that implies that his product may not be compliant. [...]In summary, SOTA should be derived from the relevant information that is provided by (harmonized) standards, guidelines, and scientific literature.A company-specific definition of SOTA, which fits to your products, is even more important than a general definition. Describe in the relevant processes how deep the research should be, which alternative methods should be included, and which are justifiably excluded. A clearly regulated approach is strongly recommended, as without limitations SOTA can become a bottomless pit. This will also help you in the context of the TD reviews – and save you one or two queries by your Notified Body.
In practice, we see that SOTA is often researched at a much later stage: The product has been on the market for a long time, and we are, for example, commissioned to research SOTA "incidentally" as part of a clinical evaluation/performance evaluation. Basically, this is possible, but it is not sufficient to consider SOTA only in this context. We have created a chart to make the impact of SOTA on the entire product life cycle tangible:Click on the graphic to enlarge
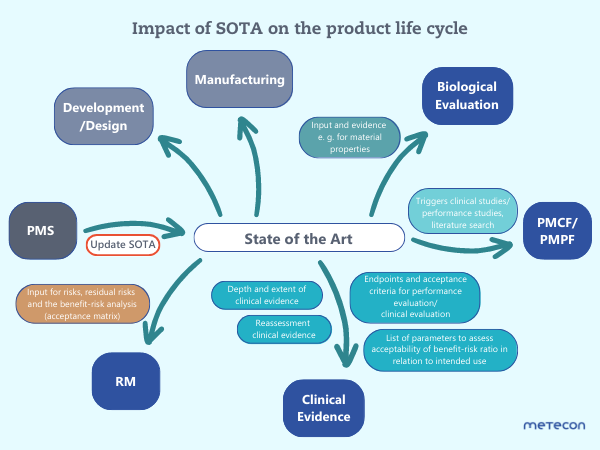
As can be seen in the chart, SOTA also influences risk management: this provides input for the acceptance criteria and the mitigation of risks. In addition, SOTA defines the criteria for the benefit-risk ratio and regularly verifies the risk analysis’ acceptance matrix. SOTA significantly influences the depth and scope of the clinical evidence (clinical evaluation plan/performance evaluation plan). For clinical evaluation/performance evaluation, SOTA provides important information on endpoints, acceptance criteria, parameters to be tested, and scientific validity for IVDs. Furthermore, SOTA influences the biological evaluation and provides relevant information – for example, on material properties and areas of use. The PMCF/PMPF of your medical devices/IVDs is also influenced by SOTA: for example, it can trigger clinical trials/performance studies or literature searches, which is documented in the PMCF/PMPF plan.We recommend: If the product is on the market, SOTA should be updated regularly as part of the PMS activities. Define the research for the update of SOTA in your PMS plan – then analyze and evaluate the results in your PMS report or PSUR (depending on the product class). In terms of development/design and manufacturing, ask yourself, for example, if there are now materials or manufacturing processes available that are more suitable for your product. These aspects should also be considered in the further development of your products as well as in the development of follow-up products.
Best Regards
 Dr. Sandra Reuter
Dr. Sandra Reuter
 Katharina Thievessen
Katharina Thievessen
Our customers’ feedback confirms that SOTA is focused by the notified bodies in the TD reviews. Among other things, they want to know how the creation and updating of SOTA is implemented and whether SOTA is included in the consideration of the benefit-risk ratio. This article explains what this means for you as a manufacturer of medical devices and (in vitro diagnostics (IVDs).
How is SOTA defined for medical devices and IVDs?
Before moving on to the implementation of the MDR/IVDR‘s requirements regarding SOTA, let's take a look at the definition of SOTA: You can´t find a definition of SOTA in Regulation (EU) 2017/745 (MDR) nor in Regulation (EU) 2017/746 (IVDR). Thereby, the guidelines MDCG 2020-1 as well as MDCG 2022-2 give more information. Both guidelines are based on the definition from the IMDRF/GRRP WG/N47 FINAL:2018 and define SOTA as follows:State of the art:Developed stage of current technical capability and/or accepted clinical practice in regard to products, processes and patient management, based on the relevant consolidated findings of science, technology and experience.
Note: The state-of-the-art embodies what is currently and generally accepted as good practice in technology and medicine. The state-of-the-art does not necessarily imply the most technologically advanced solution. The state-of- the-art described here is sometimes referred to as the "generally acknowledged state-of-the-art"
Furthermore, MEDDEV 2.7/1 rev. 4 (2016) also includes information on what SOTA means in the context of clinical evaluation:The current knowledge/ state of the art in the corresponding medical field, such as applicable standards and guidance documents, information relating to the medical condition managed with the device and its natural course, benchmark devices, other devices and medical alternatives available to the target population.In addition, the "Blue Guide" (Guide for the implementation of the EU product regulations 2016) shows which assumptions are made about SOTA in the context of the presumption of conformity:The concept of essential requirements is based on the assumption that the harmonised standards reflect generally acknowledgeable state of the art and the ESO review standards regularly. [...]Furthermore, even if the manufacturer has not used harmonised standards, a change in the relevant harmonised standard could mean a change in the state of the art that implies that his product may not be compliant. [...]In summary, SOTA should be derived from the relevant information that is provided by (harmonized) standards, guidelines, and scientific literature.A company-specific definition of SOTA, which fits to your products, is even more important than a general definition. Describe in the relevant processes how deep the research should be, which alternative methods should be included, and which are justifiably excluded. A clearly regulated approach is strongly recommended, as without limitations SOTA can become a bottomless pit. This will also help you in the context of the TD reviews – and save you one or two queries by your Notified Body.
What are the MDR‘s and IVDR‘s requirements for SOTA?
There is no clear definition of SOTA in the MDR/IVDR and no specific document is required to do so. However, both regulations contain various references to SOTA. These can be found, for example, in the essential safety and performance requirements (GSPRs):- In the GSPRs 1, 4 and 17.2 (MDR) as well as in the GSPRs 1, 4, 9.1 and 16.2 (IVDR) direct reference is made to SOTA.
- GSPR 1 states that SOTA affects many areas of the product life cycle and must be analyzed at the appropriate points: product design, manufacturing, clinical evaluation/performance evaluation, and risk management.
- GSPR 4 requires that SOTA must be considered in risk control measures.
- GSPR 9.1 in the IVDR requires that SOTA needs to be considered in the performance evaluation.
- Finally, GSPR 17.2 in the MDR (16.2 in the IVDR) states that SOTA must be complied with for software products or products that incorporate software as a component.
How does SOTA affect the product life cycle?
Ideally, you should consider SOTA at the beginning of the development and update it regularly. Why do we recommend this? If SOTA is known from the stage of the product idea, it can provide crucial information regarding for example:- Development/design (Which technology, material, etc. is used?),
- Manufacturing (Which methods and machines, etc. are used?),
- Clinical evidence (Which clinical methods are established and have sufficient clinical data?), and
- Biological safety (Which materials are suitable?).
In practice, we see that SOTA is often researched at a much later stage: The product has been on the market for a long time, and we are, for example, commissioned to research SOTA "incidentally" as part of a clinical evaluation/performance evaluation. Basically, this is possible, but it is not sufficient to consider SOTA only in this context. We have created a chart to make the impact of SOTA on the entire product life cycle tangible:Click on the graphic to enlarge

As can be seen in the chart, SOTA also influences risk management: this provides input for the acceptance criteria and the mitigation of risks. In addition, SOTA defines the criteria for the benefit-risk ratio and regularly verifies the risk analysis’ acceptance matrix. SOTA significantly influences the depth and scope of the clinical evidence (clinical evaluation plan/performance evaluation plan). For clinical evaluation/performance evaluation, SOTA provides important information on endpoints, acceptance criteria, parameters to be tested, and scientific validity for IVDs. Furthermore, SOTA influences the biological evaluation and provides relevant information – for example, on material properties and areas of use. The PMCF/PMPF of your medical devices/IVDs is also influenced by SOTA: for example, it can trigger clinical trials/performance studies or literature searches, which is documented in the PMCF/PMPF plan.We recommend: If the product is on the market, SOTA should be updated regularly as part of the PMS activities. Define the research for the update of SOTA in your PMS plan – then analyze and evaluate the results in your PMS report or PSUR (depending on the product class). In terms of development/design and manufacturing, ask yourself, for example, if there are now materials or manufacturing processes available that are more suitable for your product. These aspects should also be considered in the further development of your products as well as in the development of follow-up products.
How is SOTA created and updated?
It makes sense to regularly update SOTA as part of the PMS activities (process) and as part of updating the performance evaluation/clinical evaluation to perceive changes in the market. Any relevant new information or development in the field should trigger the re-evaluation of the risk analysis and clinical evidence. The appropriate frequency for this depends on your product and the product's environment.Conclusion
The topic around state of the art (SOTA) is complex and extensive, and it is not that easy to be aware of it every time and everywhere – we know how challenging this can be for manufacturers. Create a good strategy for dealing with SOTA, as SOTA is important throughout the product lifecycle – from the initial idea to PMS. How to create a cross-departmental approach to the topic, and how to implement it in practice? We will be happy to support you with all your questions about the State of the Art in the context of the MDR/IVDR. We are also available to answer any other questions you may have about technical documentation and TD reviews.You do not need active support, but want to get a professional opinion? We have often discussed both internally and with our customers, whether SOTA should be a stand-alone document or not. As always, there are pros and cons to everything, making our answer neither yes" nor "no". What do you think about this topic? What have you decided on in your company and how do you implement a "living" SOTA? We are ready for your questions!Best Regards
Our blog posts are researched and created with the utmost care, but are only snapshots of the regulations, which are constantly changing. We do not guarantee that older content is still current or meaningful. If you are not sure whether the article you have read on this page still corresponds to the current state of regulation, please contact us: we will quickly place your topic in the current context.

IVD Expert
Regulatory Affairs & Technical Documentation

Head of Operations
Technical Documentation & Regulatory Affairs


