Software requirements are already mentioned in the IVDD, although in a general way and limited to trisomy 21. A search for the term illustrates the relevance of software today: whereas there were only four hits in the IVDD, there are now 50 in the IVDR. The IVDR finally gives software the importance it has long deserved:""in vitro diagnostic medical device" means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following (...)"The term medical device software covers numerous devices that are applied in a therapeutic or diagnostic context; against the background of digitization in health care, their importance is increasing significantly. Primarily it refers to software IN medical devices, also referred to as embedded software, but also to software AS a medical device, so-called stand-alone software.Manufacturers of medical devices and in vitro diagnostic medical devices are faced with the challenge to develop cost-efficient products of high quality that comply with the regulations. Both IVDR and MDR require manufacturers to ensure their devices’ safety and the expectations of software documentation are also high. Especially small and medium-sized businesses often experience a lack of resources and insufficient knowledge of general regulatory requirements.As your holistic service provider we guide you through all stages of your devices’ software life cycle – competent and reliable.
Definition: Medical device software
"Medical device software is software that is intended to be used, alone or in combination, for a purpose as specified in the definition of a "medical device" in the medical devices regulation or in vitro diagnostic medical devices regulation."
Source: MDCG, "Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR", 2019.Contact us
Source: MDCG, "Guidance on Qualification and Classification of Software in Regulation (EU) 2017/745 – MDR and Regulation (EU) 2017/746 – IVDR", 2019.Contact us
We are happy to support you in the following topics:
Software can be a stand-alone medical device that enables medical decisions.
Software as an IVD
Software as an IVD
Software can also be part of a whole, for example as firmware in almost any more complex medical device.
Software in the IVD
Software in the IVD
Software as a strategic success factor: AI, cybersecurity, AI Act.
Software for IVD
Software for IVD
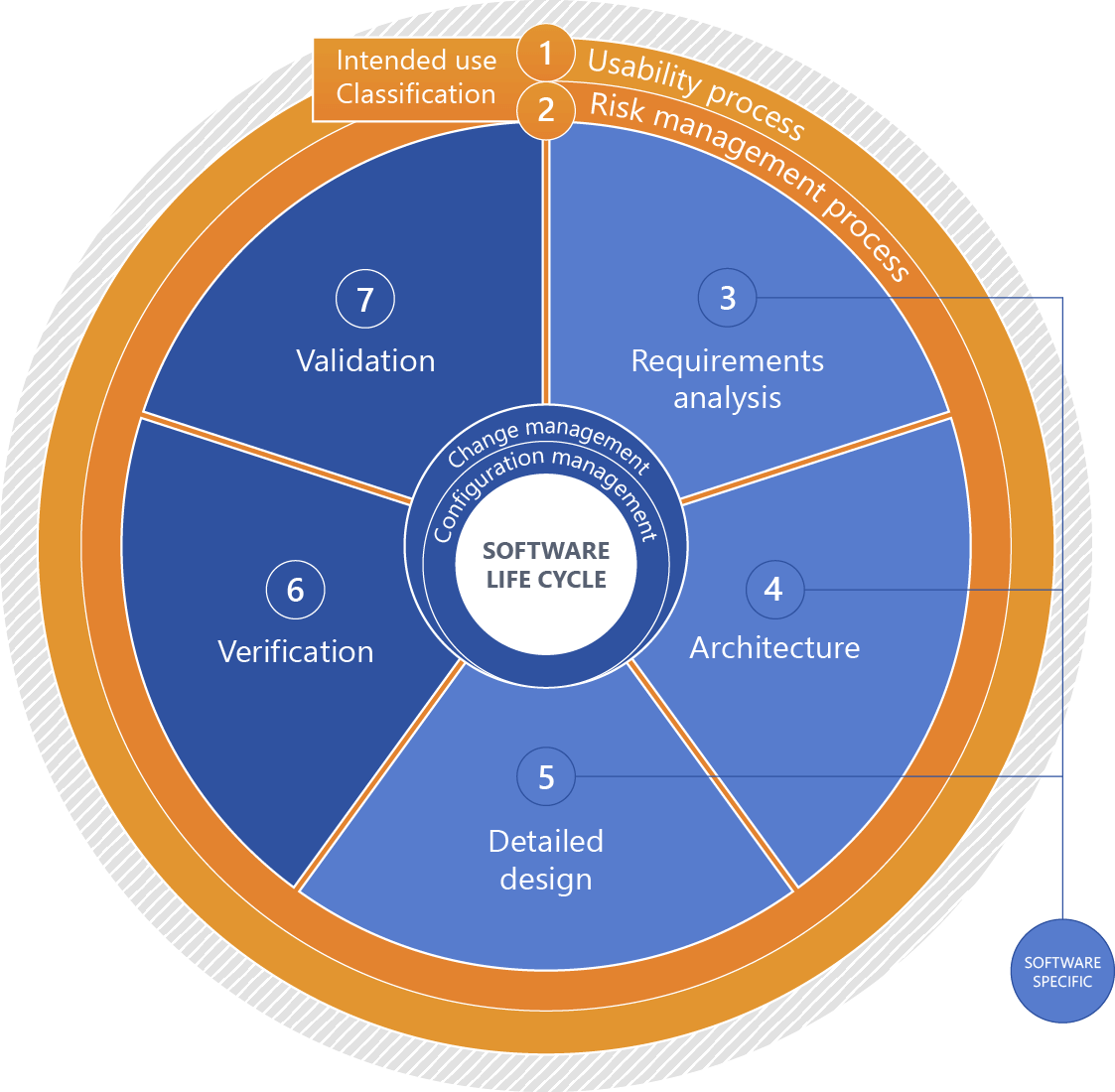
Software: Product life cycle
Please click for an explanation of the figure:
+1 - Intended use
Intended use of the IVD medical device
+2 - Classification (software safety classification)
Software classification based on the risks associated with the software considering measures of risk governance implemented outside the software with a focus on expectable damage.
+3 - Requirements analysis
Systematically deriving the software requirements from the system requirements. For stand-alone software, the software requirements are identical to the system requirements.
+4 - Architecture
Organizational structure of a system or a component (IEC 62034).
+5 - Detailed design
Detailed continuation of the system architecture to a specific solution for implementation.
+6 - Verification
Confirmation, through provision of objective evidence, that specified requirements have been fulfilled. (IEC 62304).
+7 - Validation
Confirmation, through the provision of objective evidence, that the requirements for a specific intended use or application have been fulfilled (IEC 82304-1).
Regulatory requirements for medical software
Safety requirements
Safety: Protection against device malfunctions due to software failures,Security: Protection of the software against hacker attacks and attacks from outside,Privacy: Compliance with the requirements in terms of data privacy.
Contact us
Contact us
The regulatory requirements are derived from the Regulation (EU) 2017/746 (IVDR). The general safety and performance requirements in Annex I, Chapter 2 define that a software life cycle must be established; it is adequately reflected in IEC 62304.However, IEC 62304 does not provide answers to all questions. For example, it does not regulate the validation of a device or the electrical safety of devices with embedded software. In addition, other standards such as IEC 61010-1 and IEC 61010-2 or IEC 62366-1 and IEC 82304-1, also need to be considered.The next obstacle: Although the standards mentioned above reflect the current state of the art, not all of them are necessarily harmonized for IVDR, It is therefore always recommended to consult with your notified body before applying non-harmonized standards.Software may be a stand alone medical device enabling diagnostic decisions. It may, however, also be part of a whole, for example as firmware in almost any complex medical device. And also complex production processes are virtually impossible without software today.We love challenges. How can we support you?