Digitalization in Regulatory Affairs: Optimization through Risk Management and QM Software
26/04/2022
Do you have any questions about the article or would you like to find out more about our services? We look forward to hearing from you!Make a non-binding enquiry now
In the closely monitored world of medical technology, software solutions have become essential for mastering the challenges of regulatory affairs. Various software applications support specific areas within Regulatory Affairs. The area in which software support brings the greatest benefit depends on individual workflows and processes, the level of digitization, and the size of the company. Here, we would like to provide an overview of how these software solutions can facilitate your work.
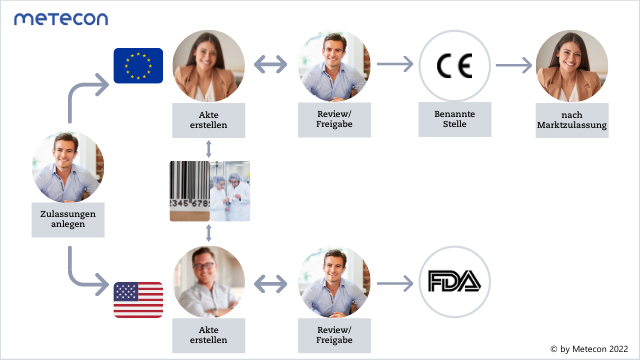
Figure 1: Simplified workflow supported by a RIM system
Their particular advantage is that artificial intelligence is already being used to search for incidents in databases due to the large volumes of data. This reduces human error and saves a lot of time.
Metecon supports you in analyzing your requirements and selecting suitable software solutions. Contact us for individual consultation and optimize your Regulatory Affairs processes for a more successful future.
Regulatory document approval
Document management systems (DMS)
On average, professionals in our industry spend more than two hours daily on filing and searching for documents. Document Management Systems (DMS) make this manual filing and searching in unstructured file folders no longer necessary. All relevant documents are centrally managed and versioned, resulting in efficient access and improved collaboration: Documents no longer circulate within the company via mail; instead, all employees, according to their permissions, have access to the relevant and current information at any time.Regulatory information management systems (RIM)
Advanced RIM systems can be used to map approval processes, implement data into actions, and – like any Regulatory Affairs software – shorten the time-to-market. Some RIM systems even allow the creation of content-based content. Regulatory Information Management Systems also offer the possibility to deposit Intended Uses once and use them in various documents through text blocks. Especially for companies with diverse product portfolios and global target markets and locations, RIM systems are of great advantage.Click on the image for full size view
Figure 1: Simplified workflow supported by a RIM system
PMS Software
Post-market surveillance is essential and needs to be both planned and executed. Various software systems exist on the market for both planning and conducting Post-Market Surveillance. While for planning PMS workflows, Regulatory Information Management Systems (RIM) can be used in principle, the execution of activities requires special applications; these can be used for creating PMS/PSUR plans and reports and also for surveys.Their particular advantage is that artificial intelligence is already being used to search for incidents in databases due to the large volumes of data. This reduces human error and saves a lot of time.
Risk management software
In the United States, approximately 4,500 drugs and medical devices are withdrawn from the market each year because their safety is not guaranteed (Source: Drugwatch). The safety of medical products is essential. Suitable risk management software minimizes possible risks throughout the entire product lifecycle and significantly supports ensuring product reliability.Quality management software (QM Software)
Compliance with quality standards is crucial – and highly time-consuming in our industry. Here too, digitization with the individually fitting tool provides relief: Quality Management Software (QM Software) can, for example, take over the entire supplier management, manage all training and qualifications of your employees, and ensure the control of documents.Conclusion
Regulatory software solutions are crucial tools to meet the challenges of medical technology. The selection and implementation of the right software are an investment in the future of any company. Through digitization, you create space for innovations and relieve yourself of administrative tasks.Metecon supports you in analyzing your requirements and selecting suitable software solutions. Contact us for individual consultation and optimize your Regulatory Affairs processes for a more successful future.
Our blog posts are researched and created with the utmost care, but are only snapshots of the regulations, which are constantly changing. We do not guarantee that older content is still current or meaningful. If you are not sure whether the article you have read on this page still corresponds to the current state of regulation, please contact us: we will quickly place your topic in the current context.


