Post-Market Surveillance Data in Clinical Evaluation - and the Correct Sequence
13/02/2024
Do you have any questions about the article or would you like to find out more about our services? We look forward to hearing from you!Make a non-binding enquiry now
Updating regulatory documents such as the Clinical Evaluation Report (CER) is a demanding, multidisciplinary task with interdependent activities. It is an obvious motivation to update every report, including references to other reports that should also be updated. Often, report templates are designed in such a way that they reference each other in an unorganized way, and the resulting release loop cannot be solved by a logical sequence.In this blog, we put the reader in the position to write the Post-Market Surveillance (PMS) section of the Clinical Evaluation Report (CER). We will explain what content needs to be considered and which other reports need to be updated before or after the CER.
Medical Device Regulation (EU) 2017/745 (MDR) , the relevant MDCG documents (including MDCG 2020-13 Clinical Evaluation Assessment Report Template/CEAR), and MEDDEV 2.7/1 , repeatedly reveals the instruction to use data from PMS and Post-Market Clinical Follow-up (PMCF) in the Clinical Evaluation, and conversely, to use clinical results in PMS reporting. To make it easier for you to write the PMS section in the Clinical Evaluation, we have extracted for you the required contents, their mentioned location, and their movement between the reports, from the regulations of the MDR and MDCG 2020-6 (legacy devices), MDCG2020-7 (PMCF-Plan), MDCG2020-8 (PMCF-Report), MDCG 2020-13 (CEAR), as well as MEDDEV 2.7/1 (Clinical Evaluation ) and 2.12/1 (Vigilance) .
This does not result in a contradiction if you pay close attention to the wording: reports are not mentioned as sources. Therefore, write - or rather complete - your reports in this sequence:
This fulfills MDCG 2020-13 in section A: "When the CER has been updated verify that this update corresponds to the most recently updated PMS/PMCF reports" without any problems referencing released documents.Please ensure that all necessary updates are carried out within a tight time frame so that the intervals between obtaining the data (e.g. literature search) and reporting are not too long (a maximum of 6 months).
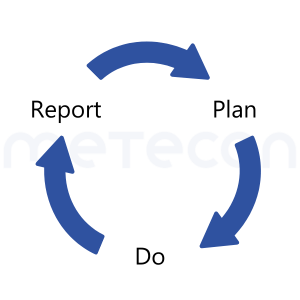 So, if we are in the updating phase, we have reached the end of the Do phase! Therefore, the results should first be summarized in the report. Deviations from the Clinical Evaluation Plan, e.g., in in key terms for literature search, can be disclosed in the Clinical Evaluation Report and explained by reacting to new information. And if data emerges differently than planned, it will not be ignored in the report.Once all analyses and conclusions from the PSUR and CER are available, the Clinical Evaluation Plan can respond to and be updated if necessary - just as the PMCF plan is now updated based on the Clinical Evaluation Report.Then the whole process starts over with the next Do period of activities according to the planning.
So, if we are in the updating phase, we have reached the end of the Do phase! Therefore, the results should first be summarized in the report. Deviations from the Clinical Evaluation Plan, e.g., in in key terms for literature search, can be disclosed in the Clinical Evaluation Report and explained by reacting to new information. And if data emerges differently than planned, it will not be ignored in the report.Once all analyses and conclusions from the PSUR and CER are available, the Clinical Evaluation Plan can respond to and be updated if necessary - just as the PMCF plan is now updated based on the Clinical Evaluation Report.Then the whole process starts over with the next Do period of activities according to the planning.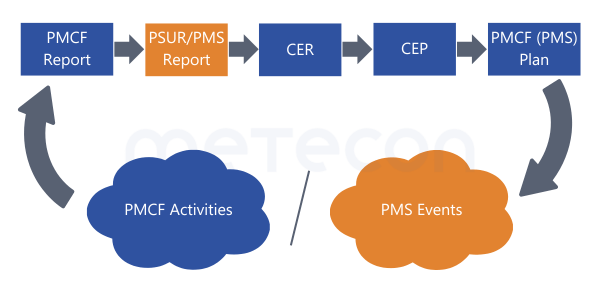 We know that, in practice, individual solutions are always needed to implement the requirements of the MDR as efficiently as possible and in line with adjacent processes. Therefore, we not only consider alternatives in the sequence of activities but also a flexible design of documentation.For example, if separate documents are maintained for CEP, literature search plan, CDP (Clinical Development Plan), and PMCF plan, the CEP can be designed so that it does not describe specific activities. It then represents a top-level plan and describes what needs to be evaluated and considered. This way, the CEP can be updated any time when needed without a chain reaction.
We know that, in practice, individual solutions are always needed to implement the requirements of the MDR as efficiently as possible and in line with adjacent processes. Therefore, we not only consider alternatives in the sequence of activities but also a flexible design of documentation.For example, if separate documents are maintained for CEP, literature search plan, CDP (Clinical Development Plan), and PMCF plan, the CEP can be designed so that it does not describe specific activities. It then represents a top-level plan and describes what needs to be evaluated and considered. This way, the CEP can be updated any time when needed without a chain reaction.
Do you need assistance with creating the clinical evaluation or with post-market surveillance? Or are you currently dealing with another complex issue in the areas of Clinical Affairs or Technical Documentation and are therefore looking for experienced professionals? Feel free to contact us at any time. Together, we will find the best solution for your specific issue.
What are the formal requirements?
A glance into theRequired Content
- MDR and MDCG expect clinical data from PMS/PMCF, significant complaints, trends, and vigilance cases.
- MEDDEV expects new clinical data, new insights, and details on all field safety corrective actions, CE and market status, since when, in which regions/countries, and sales volumes.
Data Sources to Consider
- MDR and MDCG mention PMS and PMCF activities, as well as reports on complaints and incidents.
- MEDDEV mentions PMCF studies, PMS reports, vigilance and trend reports, as well as reports on incidents and complaints to the manufacturer.
What sequence is recommended for the reports?
There are two crucial passages in the MDR regarding the relationships between PSUR, PMS, PMCF, and CER:- Article 86(1) states that the PSUR contains the results and conclusions following the PMS plan (and thus also those following the PMCF plan, which is part of the PMS plan).
- Article 61(11) states that the documentation of the Clinical Evaluation is updated based on the clinical data following the PMCF plan and PMS plan.
This does not result in a contradiction if you pay close attention to the wording: reports are not mentioned as sources. Therefore, write - or rather complete - your reports in this sequence:
- PMCF-Report
- PSUR (now considering the conclusion from the PMCF report)
- CER (then considering the clinically relevant data from the PMCF report as well as the freshly printed PSUR)
This fulfills MDCG 2020-13 in section A: "When the CER has been updated verify that this update corresponds to the most recently updated PMS/PMCF reports" without any problems referencing released documents.Please ensure that all necessary updates are carried out within a tight time frame so that the intervals between obtaining the data (e.g. literature search) and reporting are not too long (a maximum of 6 months).
Updating the Report Before the Plan?
Sounds surprising at first, doesn't it? In practice, the usual procedure is the other way around, i.e., when an update is pending, the Clinical Evaluation Plan is first updated, followed by the Clinical Evaluation Report. This is because typical templates provide for updating the Clinical Evaluation Plan according to the new findings in PMS.But let's consider whether this actually (always) has to be the case. Let's remember the fundamental sequence of every activity: Plan - Do - Report. So, if we are in the updating phase, we have reached the end of the Do phase! Therefore, the results should first be summarized in the report. Deviations from the Clinical Evaluation Plan, e.g., in in key terms for literature search, can be disclosed in the Clinical Evaluation Report and explained by reacting to new information. And if data emerges differently than planned, it will not be ignored in the report.Once all analyses and conclusions from the PSUR and CER are available, the Clinical Evaluation Plan can respond to and be updated if necessary - just as the PMCF plan is now updated based on the Clinical Evaluation Report.Then the whole process starts over with the next Do period of activities according to the planning.
So, if we are in the updating phase, we have reached the end of the Do phase! Therefore, the results should first be summarized in the report. Deviations from the Clinical Evaluation Plan, e.g., in in key terms for literature search, can be disclosed in the Clinical Evaluation Report and explained by reacting to new information. And if data emerges differently than planned, it will not be ignored in the report.Once all analyses and conclusions from the PSUR and CER are available, the Clinical Evaluation Plan can respond to and be updated if necessary - just as the PMCF plan is now updated based on the Clinical Evaluation Report.Then the whole process starts over with the next Do period of activities according to the planning. We know that, in practice, individual solutions are always needed to implement the requirements of the MDR as efficiently as possible and in line with adjacent processes. Therefore, we not only consider alternatives in the sequence of activities but also a flexible design of documentation.For example, if separate documents are maintained for CEP, literature search plan, CDP (Clinical Development Plan), and PMCF plan, the CEP can be designed so that it does not describe specific activities. It then represents a top-level plan and describes what needs to be evaluated and considered. This way, the CEP can be updated any time when needed without a chain reaction.
We know that, in practice, individual solutions are always needed to implement the requirements of the MDR as efficiently as possible and in line with adjacent processes. Therefore, we not only consider alternatives in the sequence of activities but also a flexible design of documentation.For example, if separate documents are maintained for CEP, literature search plan, CDP (Clinical Development Plan), and PMCF plan, the CEP can be designed so that it does not describe specific activities. It then represents a top-level plan and describes what needs to be evaluated and considered. This way, the CEP can be updated any time when needed without a chain reaction.Need Further Assistance?
Do you need assistance with creating the clinical evaluation or with post-market surveillance? Or are you currently dealing with another complex issue in the areas of Clinical Affairs or Technical Documentation and are therefore looking for experienced professionals? Feel free to contact us at any time. Together, we will find the best solution for your specific issue.
 |
| Dr. Philippe Thiel |
| Expert Clinical Affairs |
| philippe.thiel@metecon.de |
| Connect via LinkedIn |
Our blog posts are researched and created with the utmost care, but are only snapshots of the regulations, which are constantly changing. We do not guarantee that older content is still current or meaningful. If you are not sure whether the article you have read on this page still corresponds to the current state of regulation, please contact us: we will quickly place your topic in the current context.


